Optimization of Automated Synthesis of Amide-Linked RNA
- PMID: 35721988
- PMCID: PMC9201902
- DOI: 10.1021/acsomega.2c02742
Optimization of Automated Synthesis of Amide-Linked RNA
Abstract
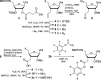
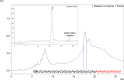
The recent FDA approval of several antisense and siRNA drugs illustrates the utility of nucleic acid chemical modifications, but numerous challenges remain for generalized nucleic acid therapeutics, urging the exploration of new modification strategies. Replacing backbone phosphates with amides has shown promise for enhancing siRNA activity, specificity, and nuclease resistance; however, amide-linked RNA has not been fully explored due to lengthy and low yielding manual amide coupling procedures. We have addressed this by automating the assembly of amide-linked RNA using an Expedite 8909 nucleic acid synthesizer and optimizing solid-phase synthesis conditions to achieve 91-95% yields in just 5 min of coupling time. The optimized protocol allowed synthesis of a 21-nucleotide-long siRNA guide strand having six consecutive amide linkages at the 3'-end with an overall yield of ∼1%. Our results show that the steric hindrance caused by bulky 2'-O protecting groups and steric hindrance of the solid support are the key optimization variables for improving the amide couplings.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Idziak I.; Just G.; Damha M. J.; Giannaris P. A. Synthesis and Hybridization Properties of Amide-Linked Thymidine Dimers Incorporated into Oligodeoxynucleotides. Tetrahedron Lett. 1993, 34, 5417–5420. 10.1016/s0040-4039(00)73923-2. - DOI
-
- De Mesmaeker A.; Waldner A.; Lebreton J.; Hoffmann P.; Fritsch V.; Wolf R. M.; Freier S. M. Amides as a new type of backbone modifications in oligonucleotides. Angew. Chem., Int. Ed. Engl. 1994, 33, 226–229. 10.1002/anie.199402261. - DOI
-
- von Matt P.; De Mesmaeker A.; Pieles U.; Zürcher W.; Altmann K.-H. 2’-deoxyribo-PNAs: a structurally novel class of polyamide nucleic acids with good RNA and DNA binding affinity. Tetrahedron Lett. 1999, 40, 2899–2902. 10.1016/s0040-4039(99)00389-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

