Urgent and emergent breast lesions - A primer for the general radiologist, on-call resident and sonographer
- PMID: 35722051
- PMCID: PMC9201204
- DOI: 10.1002/ajum.12296
Urgent and emergent breast lesions - A primer for the general radiologist, on-call resident and sonographer
Abstract
There are very few true breast emergencies. While infrequent, women do present to emergency departments or urgent care centres with breast-related concerns. In this case-based review, both common and uncommon urgent and emergent breast lesions are presented, emphasising ultrasound characteristics and imaging optimisation to improve accurate diagnosis and appropriate recommendations.
Keywords: breast abscess; breast emergencies; breast necrotising fasciitis; breast pseudoaneurysm; mastitis; nipple piercings.
© 2022 Mayo Clinic. Australasian Journal of Ultrasound in Medicine published by John Wiley & Sons Australia, Ltd on behalf of Australasian Society for Ultrasound in Medicine.
Conflict of interest statement
None declared.
Figures

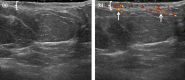

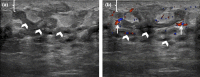

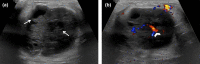
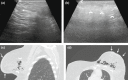
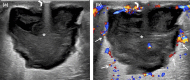
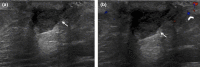


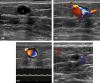


References
-
- Hines N, Leibman A, David M. Breast problems presenting in the emergency room. Emerg Radiol 2007; 14(1): 23–8. - PubMed
-
- D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI‐RADS® Atlas Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. pp. 533–9.
-
- Carpentier B, Hayward J, Strachowski L. Enhancing your acoustics: ultrasound image optimization of breast lesions. J Ultrasound Med 2017; 36(7): 1479–85. - PubMed
-
- ACR practice parameter for the performance of ultrasound‐guided percutaneous breast interventional procedures, (n.d.). Available from: https://www.acr.org/‐/media/ACR/Files/Practice‐Parameters/US‐GuidedBreas.... Retrieved 26 Mar 2020.
-
- Mendelson EB, Böhm‐Vélez M, Berg WA, Whitman GJ, Feldman MI, Madjar WA, et al. ACR BI‐RADS® Ultrasound. ACR BI‐RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
