Identification and Functional Characterization of Peptides With Antimicrobial Activity From the Syphilis Spirochete, Treponema pallidum
- PMID: 35722306
- PMCID: PMC9200625
- DOI: 10.3389/fmicb.2022.888525
Identification and Functional Characterization of Peptides With Antimicrobial Activity From the Syphilis Spirochete, Treponema pallidum
Abstract
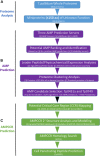
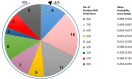
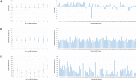
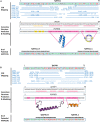
The etiological agent of syphilis, Treponema pallidum ssp. pallidum, is a highly invasive "stealth" pathogen that can evade the host immune response and persist within the host for decades. This obligate human pathogen is adept at establishing infection and surviving at sites within the host that have a multitude of competing microbes, sometimes including pathogens. One survival strategy employed by bacteria found at polymicrobial sites is elimination of competing microorganisms by production of antimicrobial peptides (AMPs). Antimicrobial peptides are low molecular weight proteins (miniproteins) that function directly via inhibition and killing of microbes and/or indirectly via modulation of the host immune response, which can facilitate immune evasion. In the current study, we used bioinformatics to show that approximately 7% of the T. pallidum proteome is comprised of miniproteins of 150 amino acids or less with unknown functions. To investigate the possibility that AMP production is an unrecognized defense strategy used by T. pallidum during infection, we developed a bioinformatics pipeline to analyze the complement of T. pallidum miniproteins of unknown function for the identification of potential AMPs. This analysis identified 45 T. pallidum AMP candidates; of these, Tp0451a and Tp0749 were subjected to further bioinformatic analyses to identify AMP critical core regions (AMPCCRs). Four potential AMPCCRs from the two predicted AMPs were identified and peptides corresponding to these AMPCCRs were experimentally confirmed to exhibit bacteriostatic and bactericidal activity against a panel of biologically relevant Gram-positive and Gram-negative bacteria. Immunomodulation assays performed under inflammatory conditions demonstrated that one of the AMPCCRs was also capable of differentially regulating expression of two pro-inflammatory chemokines [monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8)]. These findings demonstrate proof-of-concept for our developed AMP identification pipeline and are consistent with the novel concept that T. pallidum expresses AMPs to defend against competing microbes and modulate the host immune response.
Keywords: Treponema pallidum; antimicrobial peptides; bactericidal; bacteriostatic; syphilis.
Copyright © 2022 Houston, Schovanek, Conway, Mustafa, Gomez, Ramaswamy, Haimour, Boulanger, Reynolds and Cameron.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Syphilis and the host: multi-omic analysis of host cellular responses to Treponema pallidum provides novel insight into syphilis pathogenesis.Front Microbiol. 2023 Sep 19;14:1254342. doi: 10.3389/fmicb.2023.1254342. eCollection 2023. Front Microbiol. 2023. PMID: 37795301 Free PMC article.
-
Characterizing the Syphilis-Causing Treponema pallidum ssp. pallidum Proteome Using Complementary Mass Spectrometry.PLoS Negl Trop Dis. 2016 Sep 8;10(9):e0004988. doi: 10.1371/journal.pntd.0004988. eCollection 2016 Sep. PLoS Negl Trop Dis. 2016. PMID: 27606673 Free PMC article.
-
Polypeptides of Treponema pallidum: progress toward understanding their structural, functional, and immunologic roles. Treponema Pallidum Polypeptide Research Group.Microbiol Rev. 1993 Sep;57(3):750-79. doi: 10.1128/mr.57.3.750-779.1993. Microbiol Rev. 1993. PMID: 8246847 Free PMC article. Review.
-
Identification of the Neuroinvasive Pathogen Host Target, LamR, as an Endothelial Receptor for the Treponema pallidum Adhesin Tp0751.mSphere. 2020 Apr 1;5(2):e00195-20. doi: 10.1128/mSphere.00195-20. mSphere. 2020. PMID: 32238570 Free PMC article.
-
Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen.Nat Rev Microbiol. 2016 Dec;14(12):744-759. doi: 10.1038/nrmicro.2016.141. Epub 2016 Oct 10. Nat Rev Microbiol. 2016. PMID: 27721440 Free PMC article. Review.
Cited by
-
Syphilis and the host: multi-omic analysis of host cellular responses to Treponema pallidum provides novel insight into syphilis pathogenesis.Front Microbiol. 2023 Sep 19;14:1254342. doi: 10.3389/fmicb.2023.1254342. eCollection 2023. Front Microbiol. 2023. PMID: 37795301 Free PMC article.
-
Establishment of the Nichols strain as the type strain of Treponema pallidum.Int J Syst Evol Microbiol. 2025 Feb;75(2):006697. doi: 10.1099/ijsem.0.006697. Int J Syst Evol Microbiol. 2025. PMID: 40014041 Free PMC article. Review.
-
Advances in the Role of Antimicrobial Peptides in the Management of Sexually Transmitted Infections.J Clin Lab Anal. 2025 Jun;39(11):e70041. doi: 10.1002/jcla.70041. Epub 2025 May 14. J Clin Lab Anal. 2025. PMID: 40366090 Free PMC article. Review.
-
Proteomic analysis of the Treponema pallidum subsp. pallidum SS14 strain: coverage and comparison with the Nichols strain proteome.Front Microbiol. 2024 Dec 11;15:1505893. doi: 10.3389/fmicb.2024.1505893. eCollection 2024. Front Microbiol. 2024. PMID: 39723147 Free PMC article.
-
In-Depth Proteome Coverage of In Vitro-Cultured Treponema pallidum and Quantitative Comparison Analyses with In Vivo-Grown Treponemes.J Proteome Res. 2024 May 3;23(5):1725-1743. doi: 10.1021/acs.jproteome.3c00891. Epub 2024 Apr 18. J Proteome Res. 2024. PMID: 38636938 Free PMC article.
References
-
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215 403–410. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

