Molecular, Viral and Clinical Features of Alcohol- and Non-Alcohol-Induced Liver Injury
- PMID: 35723310
- PMCID: PMC8947098
- DOI: 10.3390/cimb44030087
Molecular, Viral and Clinical Features of Alcohol- and Non-Alcohol-Induced Liver Injury
Abstract
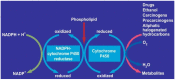
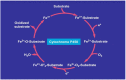
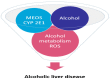
Hepatic cells are sensitive to internal and external signals. Ethanol is one of the oldest and most widely used drugs in the world. The focus on the mechanistic engine of the alcohol-induced injury has been in the liver, which is responsible for the pathways of alcohol metabolism. Ethanol undergoes a phase I type of reaction, mainly catalyzed by the cytoplasmic enzyme, alcohol dehydrogenase (ADH), and by the microsomal ethanol-oxidizing system (MEOS). Reactive oxygen species (ROS) generated by cytochrome (CYP) 2E1 activity and MEOS contribute to ethanol-induced toxicity. We aimed to: (1) Describe the cellular, pathophysiological and clinical effects of alcohol misuse on the liver; (2) Select the biomarkers and analytical methods utilized by the clinical laboratory to assess alcohol exposure; (3) Provide therapeutic ideas to prevent/reduce alcohol-induced liver injury; (4) Provide up-to-date knowledge regarding the Corona virus and its affect on the liver; (5) Link rare diseases with alcohol consumption. The current review contributes to risk identification of patients with alcoholic, as well as non-alcoholic, liver disease and metabolic syndrome. Additional prevalence of ethnic, genetic, and viral vulnerabilities are presented.
Keywords: alcoholic liver disease; apoptosis; cellular toxicity; cytochrome P450; cytokines; fibrosis; inflammation; microsomal ethanol oxidizing system; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Microsomal Ethanol-Oxidizing System: Success Over 50 Years and an Encouraging Future.Alcohol Clin Exp Res. 2019 Mar;43(3):386-400. doi: 10.1111/acer.13961. Epub 2019 Feb 11. Alcohol Clin Exp Res. 2019. PMID: 30667528 Review.
-
Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects.Biomedicines. 2018 Nov 12;6(4):106. doi: 10.3390/biomedicines6040106. Biomedicines. 2018. PMID: 30424581 Free PMC article. Review.
-
Alcoholic Liver Disease: Current Mechanistic Aspects with Focus on Their Clinical Relevance.Biomedicines. 2019 Sep 5;7(3):68. doi: 10.3390/biomedicines7030068. Biomedicines. 2019. PMID: 31491888 Free PMC article. Review.
-
Cytochrome P450s and Alcoholic Liver Disease.Curr Pharm Des. 2018;24(14):1502-1517. doi: 10.2174/1381612824666180410091511. Curr Pharm Des. 2018. PMID: 29637855 Free PMC article. Review.
-
Alcohol, microbiome, life style influence alcohol and non-alcoholic organ damage.Exp Mol Pathol. 2017 Feb;102(1):162-180. doi: 10.1016/j.yexmp.2017.01.003. Epub 2017 Jan 7. Exp Mol Pathol. 2017. PMID: 28077318 Review.
Cited by
-
Protective Effects of Lycium ruthenicum Murray against Acute Alcoholic Liver Disease in Mice via the Nrf2/HO-1/NF-κB Signaling Pathway.Pharmaceuticals (Basel). 2024 Apr 13;17(4):497. doi: 10.3390/ph17040497. Pharmaceuticals (Basel). 2024. PMID: 38675458 Free PMC article.
-
Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different.Int J Mol Sci. 2022 Dec 19;23(24):16226. doi: 10.3390/ijms232416226. Int J Mol Sci. 2022. PMID: 36555867 Free PMC article. Review.
-
Pathogenesis of Alcoholic Fatty Liver a Narrative Review.Life (Basel). 2023 Jul 30;13(8):1662. doi: 10.3390/life13081662. Life (Basel). 2023. PMID: 37629519 Free PMC article. Review.
-
Network medicine-based analysis of the hepatoprotective effects of Amomum villosum Lour. on alcoholic liver disease in rats.Food Sci Nutr. 2024 Feb 22;12(5):3759-3773. doi: 10.1002/fsn3.4046. eCollection 2024 May. Food Sci Nutr. 2024. PMID: 38726425 Free PMC article.
-
The Preparation of Black Goji Berry Enzyme and Its Therapeutic Effect on Alcoholic Liver Injury in Mice.Foods. 2025 Feb 6;14(3):523. doi: 10.3390/foods14030523. Foods. 2025. PMID: 39942116 Free PMC article.
References
-
- Lieber C.S., DeCarli L.M. The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J. Pharmacol. Exp. Ther. 1972;181:279–287. - PubMed
-
- Lieber C.S., DeCarli L.M., Matsuzaki S., Ohnishi K., Teschke R. Methods in Enzymology. Volume 52. Elsevier BV; Amsterdam, The Netherlands: 1978. The Microsomal ethanol oxidizing systems (MEOS) pp. 355–367. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

