Proteomic Changes to the Updated Discovery of Engineered Insulin and Its Analogs: Pros and Cons
- PMID: 35723344
- PMCID: PMC8929101
- DOI: 10.3390/cimb44020059
Proteomic Changes to the Updated Discovery of Engineered Insulin and Its Analogs: Pros and Cons
Abstract
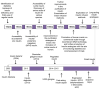
The destruction of β-cells of the pancreas leads to either insulin shortage or the complete absence of insulin, which in turn causes diabetes Mellitus. For treating diabetes, many trials have been conducted since the 19th century until now. In ancient times, insulin from an animal's extract was taken to treat human beings. However, this resulted in some serious allergic reactions. Therefore, scientists and researchers have tried their best to find alternative ways for managing diabetes with progressive advancements in biotechnology. However, a lot of research trials have been conducted, and they discovered more progressed strategies and approaches to treat type I and II diabetes with satisfaction. Still, investigators are finding more appropriate ways to treat diabetes accurately. They formulated insulin analogs that mimic the naturally produced human insulin through recombinant DNA technology and devised many methods for appropriate delivery of insulin. This review will address the following questions: What is insulin preparation? How were these devised and what are the impacts (both positive and negative) of such insulin analogs against TIDM (type-I diabetes mellitus) and TIIDM (type-II diabetes mellitus)? This review article will also demonstrate approaches for the delivery of insulin analogs into the human body and some future directions for further improvement of insulin treatment.
Keywords: diabetes mellitus; hemoglobin A1C; hypoglycemia; insulin; insulin analogs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
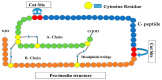
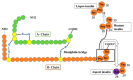
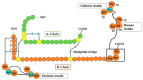
- Steiner D., Rouille Y., Gong Q., Martin S., Carroll R., Chan S. The role of prohormone convertases in insulin biosynthesis: Evidence for inherited defects in their action in man and experimental animals. Diabetes Metab. 1996;22:94–104. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

