A native chemical chaperone in the human eye lens
- PMID: 35723573
- PMCID: PMC9246369
- DOI: 10.7554/eLife.76923
A native chemical chaperone in the human eye lens
Abstract
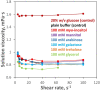
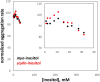
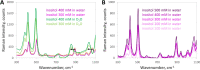
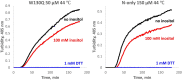
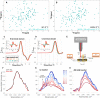
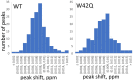
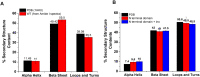
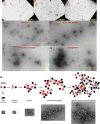
Cataract is one of the most prevalent protein aggregation disorders and still the most common cause of vision loss worldwide. The metabolically quiescent core region of the human lens lacks cellular or protein turnover; it has therefore evolved remarkable mechanisms to resist light-scattering protein aggregation for a lifetime. We now report that one such mechanism involves an unusually abundant lens metabolite, myo-inositol, suppressing aggregation of lens crystallins. We quantified aggregation suppression using our previously well-characterized in vitro aggregation assays of oxidation-mimicking human γD-crystallin variants and investigated myo-inositol's molecular mechanism of action using solution NMR, negative-stain TEM, differential scanning fluorometry, thermal scanning Raman spectroscopy, turbidimetry in redox buffers, and free thiol quantitation. Unlike many known chemical chaperones, myo-inositol's primary target was not the native, unfolded, or final aggregated states of the protein; rather, we propose that it was the rate-limiting bimolecular step on the aggregation pathway. Given recent metabolomic evidence that it is severely depleted in human cataractous lenses compared to age-matched controls, we suggest that maintaining or restoring healthy levels of myo-inositol in the lens may be a simple, safe, and globally accessible strategy to prevent or delay lens opacification due to age-onset cataract.
Keywords: biochemistry; cataract; chemical biology; chemical chaperone; crystallin; disulfide; eye lens; none; protein aggregation.
© 2022, Serebryany et al.
Conflict of interest statement
ES, SC, CW, DT, NW, AM, RK, ES No competing interests declared
Figures





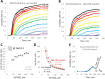
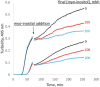

Similar articles
-
Cataract-causing defect of a mutant γ-crystallin proceeds through an aggregation pathway which bypasses recognition by the α-crystallin chaperone.PLoS One. 2012;7(5):e37256. doi: 10.1371/journal.pone.0037256. Epub 2012 May 24. PLoS One. 2012. PMID: 22655036 Free PMC article.
-
Proteostasis and the Regulation of Intra- and Extracellular Protein Aggregation by ATP-Independent Molecular Chaperones: Lens α-Crystallins and Milk Caseins.Acc Chem Res. 2018 Mar 20;51(3):745-752. doi: 10.1021/acs.accounts.7b00250. Epub 2018 Feb 14. Acc Chem Res. 2018. PMID: 29442498 Review.
-
Structural and functional properties, chaperone activity and posttranslational modifications of alpha-crystallin and its related subunits in the crystalline lens: N-acetylcarnosine, carnosine and carcinine act as alpha- crystallin/small heat shock protein enhancers in prevention and dissolution of cataract in ocular drug delivery formulations of novel therapeutic agents.Recent Pat Drug Deliv Formul. 2012 Aug;6(2):107-48. doi: 10.2174/187221112800672976. Recent Pat Drug Deliv Formul. 2012. PMID: 22436026 Review.
-
Modulation of alpha-crystallin chaperone activity: a target to prevent or delay cataract?IUBMB Life. 2009 May;61(5):485-95. doi: 10.1002/iub.176. IUBMB Life. 2009. PMID: 19391162 Review.
-
Crystallins and Their Complexes.Subcell Biochem. 2019;93:439-460. doi: 10.1007/978-3-030-28151-9_14. Subcell Biochem. 2019. PMID: 31939160 Review.
Cited by
-
Role of Oxidative Stress in Ocular Diseases: A Balancing Act.Metabolites. 2023 Jan 27;13(2):187. doi: 10.3390/metabo13020187. Metabolites. 2023. PMID: 36837806 Free PMC article. Review.
-
The Functional Significance of High Cysteine Content in Eye Lens γ-Crystallins.Biomolecules. 2024 May 17;14(5):594. doi: 10.3390/biom14050594. Biomolecules. 2024. PMID: 38786000 Free PMC article. Review.
-
Redox chemistry of lens crystallins: A system of cysteines.Exp Eye Res. 2021 Oct;211:108707. doi: 10.1016/j.exer.2021.108707. Epub 2021 Jul 29. Exp Eye Res. 2021. PMID: 34332989 Free PMC article. Review.
-
Aging of the eye: Lessons from cataracts and age-related macular degeneration.Ageing Res Rev. 2024 Aug;99:102407. doi: 10.1016/j.arr.2024.102407. Epub 2024 Jul 6. Ageing Res Rev. 2024. PMID: 38977082 Free PMC article. Review.
-
Insights into the biochemical and biophysical mechanisms mediating the longevity of the transparent optics of the eye lens.J Biol Chem. 2022 Nov;298(11):102537. doi: 10.1016/j.jbc.2022.102537. Epub 2022 Sep 27. J Biol Chem. 2022. PMID: 36174677 Free PMC article. Review.
References
-
- Basak A, Bateman O, Slingsby C, Pande A, Asherie N, Ogun O, Benedek GB, Pande J. High-resolution X-ray crystal structures of human gammaD crystallin (1.25 A) and the R58H mutant (1.15 A) associated with aculeiform cataract. Journal of Molecular Biology. 2003;328:1137–1147. doi: 10.1016/s0022-2836(03)00375-9. - DOI - PubMed
-
- Beg I, Minton AP, Islam A, Hassan MI, Ahmad F. The pH Dependence of Saccharides’ Influence on Thermal Denaturation of Two Model Proteins Supports an Excluded Volume Model for Stabilization Generalized to Allow for Intramolecular Electrostatic Interactions. The Journal of Biological Chemistry. 2017;292:505–511. doi: 10.1074/jbc.M116.757302. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

