IL27 Signaling Serves as an Immunologic Checkpoint for Innate Cytotoxic Cells to Promote Hepatocellular Carcinoma
- PMID: 35723626
- PMCID: PMC9357073
- DOI: 10.1158/2159-8290.CD-20-1628
IL27 Signaling Serves as an Immunologic Checkpoint for Innate Cytotoxic Cells to Promote Hepatocellular Carcinoma
Abstract
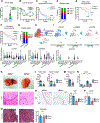
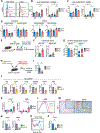
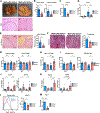
Although inflammatory mechanisms driving hepatocellular carcinoma (HCC) have been proposed, the regulators of anticancer immunity in HCC remain poorly understood. We found that IL27 receptor (IL27R) signaling promotes HCC development in vivo. High IL27EBI3 cytokine or IL27RA expression correlated with poor prognosis for patients with HCC. Loss of IL27R suppressed HCC in vivo in two different models of hepatocarcinogenesis. Mechanistically, IL27R sig-naling within the tumor microenvironment restrains the cytotoxicity of innate cytotoxic lymphocytes. IL27R ablation enhanced their accumulation and activation, whereas depletion or functional impairment of innate cytotoxic cells abrogated the effect of IL27R disruption. Pharmacologic neutralization of IL27 signaling increased infiltration of innate cytotoxic lymphocytes with upregulated cytotoxic molecules and reduced HCC development. Our data reveal an unexpected role of IL27R signaling as an immunologic checkpoint regulating innate cytotoxic lymphocytes and promoting HCC of different etiologies, thus indicating a therapeutic potential for IL27 pathway blockade in HCC.
Significance: HCC, the most common form of liver cancer, is characterized by a poor survival rate and limited treatment options. The discovery of a novel IL27-dependent mechanism controlling anticancer cytotoxic immune response will pave the road for new treatment options for this devastating disease. This article is highlighted in the In This Issue feature, p. 1825.
©2022 American Association for Cancer Research.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- ZIA BC010877/ImNIH/Intramural NIH HHS/United States
- R01 CA273925/CA/NCI NIH HHS/United States
- ZIA BC010876/ImNIH/Intramural NIH HHS/United States
- R01 CA227629/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- ZIA BC010313/ImNIH/Intramural NIH HHS/United States
- R37 AI110985/AI/NIAID NIH HHS/United States
- R50 CA211199/CA/NCI NIH HHS/United States
- Z01 BC010313/ImNIH/Intramural NIH HHS/United States
- R01 CA218133/CA/NCI NIH HHS/United States
- ZIA BC011870/ImNIH/Intramural NIH HHS/United States
- R21 CA202396/CA/NCI NIH HHS/United States
- R01 HL149946/HL/NHLBI NIH HHS/United States
- Z01 BC010877/ImNIH/Intramural NIH HHS/United States
- R01 HL133669/HL/NHLBI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

