Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia
- PMID: 35723686
- PMCID: PMC9208259
- DOI: 10.1007/s00134-022-06684-3
Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia
Abstract
Purpose: Severe community-acquired pneumonia (CAP) requiring intensive care unit admission is associated with significant acute and long-term morbidity and mortality. We hypothesized that downregulation of systemic and pulmonary inflammation with prolonged low-dose methylprednisolone treatment would accelerate pneumonia resolution and improve clinical outcomes.
Methods: This double-blind, randomized, placebo-controlled clinical trial recruited adult patients within 72-96 h of hospital presentation. Patients were randomized in 1:1 ratio; an intravenous 40 mg loading bolus was followed by 40 mg/day through day 7 and progressive tapering during the 20-day treatment course. Randomization was stratified by site and need for mechanical ventilation (MV) at the time of randomization. Outcomes included a primary endpoint of 60-day all-cause mortality and secondary endpoints of morbidity and mortality up to 1 year of follow-up.
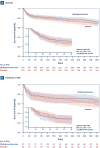
Results: Between January 2012 and April 2016, 586 patients from 42 Veterans Affairs Medical Centers were randomized, short of the 1420 target sample size because of low recruitment. 584 patients were included in the analysis. There was no significant difference in 60-day mortality between the methylprednisolone and placebo arms (16% vs. 18%; adjusted odds ratio 0.90, 95% CI 0.57-1.40). There were no significant differences in secondary outcomes or complications.
Conclusions: In patients with severe CAP, prolonged low-dose methylprednisolone treatment did not significantly reduce 60-day mortality. Treatment was not associated with increased complications.
Trial registration: ClinicalTrials.gov NCT01283009.
Keywords: Community-acquired pneumonia; Glucocorticoids; Intensive care; Methylprednisolone; Randomized clinical trial.
© 2022. This is a U.S. Goverment work and not under copyright protection in the US; foreign protection ma apply.
Conflict of interest statement
No funding or other conflicts to report.
Figures


Comment in
-
Corticosteroids for severe community-acquired pneumonia: a story without an ending.Intensive Care Med. 2022 Aug;48(8):1053-1055. doi: 10.1007/s00134-022-06699-w. Epub 2022 May 13. Intensive Care Med. 2022. PMID: 35552779 Free PMC article. No abstract available.
References
-
- Kang C-I, Song J-H, Kim SH, Chung DR, Peck KR, Thamlikitkul V, Wang H, So TM-K, Hsueh P-R, Yasin RM. Risk factors and pathogenic significance of bacteremic pneumonia in adult patients with community-acquired pneumococcal pneumonia. J Infect. 2013;66:34–40. doi: 10.1016/j.jinf.2012.08.011. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

