Celebration of a century of insulin therapy in children with type 1 diabetes
- PMID: 35725290
- PMCID: PMC9763182
- DOI: 10.1136/archdischild-2022-323975
Celebration of a century of insulin therapy in children with type 1 diabetes
Abstract
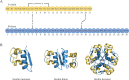
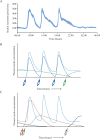
Insulin is the key anabolic hormone of metabolism, with clear effects on glycaemia. Near-complete insulin deficiency occurs in type 1 diabetes (T1D), the predominant form affecting children, and uniformly fatal until the discovery of insulin. By the early 20th century, it was known that T1D was caused by the lack of a factor from pancreatic islets, but isolation of this substance proved elusive. In 1921, an unusual team in Toronto comprising a surgeon, a medical student, a physiologist and a biochemist successfully isolated a glucose-lowering pancreatic endocrine secretion. They treated an emaciated 14-year-old boy in 1922, restoring his health and allowing him to live for another 13 years. Thus began an era of remarkable progress and partnership between academia and the pharmaceutical industry to produce drugs that benefit sick people. The Toronto team received the 1923 Nobel Prize, and more Nobel Prizes for work with insulin followed: for elucidation of its amino acid sequence and crystalline structure, and for its role in the development of radioimmunoassays to measure circulating hormone concentrations. Human insulin was the first hormone synthesised by recombinant methods, permitting modifications to enable improved absorption rates and alterations in duration of action. Coupled with delivery via insulin pens, programmable pumps and continuous glucose monitors, metabolic control and quality of life vastly improved and T1D in children was converted from uniformly fatal to a manageable chronic condition. We describe this remarkable ongoing story as insulin remains a paradigm for human ingenuity to heal nature's maladies.
Keywords: endocrinology; paediatrics; technology.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LML reports funding support for the manuscript as noted. MAS served on an advisory board for Novo Nordisk.
Figures

References
-
- Bliss M. The discovery of insulin (the Canada 150 collection. University of Toronto Press, Scholarly Publishing Division, 2007.
-
- Banting F, Best C, Collip J. The effect produced on diabetes by extractions of pancreas. Transact Ass Amer Physicians 1922;37:337.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
