Weaning of maintenance immunosuppressive therapy in lupus nephritis (WIN-Lupus): results of a multicentre randomised controlled trial
- PMID: 35725295
- PMCID: PMC9484365
- DOI: 10.1136/annrheumdis-2022-222435
Weaning of maintenance immunosuppressive therapy in lupus nephritis (WIN-Lupus): results of a multicentre randomised controlled trial
Abstract
Objectives: Lupus nephritis (LN) is a frequent complication of systemic lupus erythematosus (SLE). Severe (proliferative) forms of LN are treated with induction immunosuppressive therapy (IST), followed by maintenance IST, to target remission and avoid relapses. The optimal duration of maintenance IST is unknown. The WIN-Lupus trial tested whether IST discontinuation after 2‒3 years was non-inferior to IST continuation for two more years in proliferative LN.
Methods: WIN-Lupus was an investigator-initiated multicentre randomised controlled trial. Patients receiving maintenance IST with azathioprine or mycophenolate mofetil for 2-3 years, and hydroxychloroquine, were randomised (1:1) into two groups: (1) IST continuation and (2) IST discontinuation. The primary endpoint was the relapse rate of proliferative LN at 24 months. Main secondary endpoints were the rate of severe SLE flares, survival without renal relapse or severe flare, adverse events.
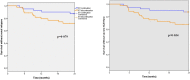
Results: Between 2011 and 2016, 96 patients (out of 200 planned) were randomised in WIN-Lupus: IST continuation group (n=48), IST discontinuation group (n=48). Relapse of proliferative LN occurred in 5/40 (12.5%) patients with IST continuation and in 12/44 (27.3%) patients with IST discontinuation (difference 14.8% (95% CI -1.9 to 31.5)). Non-inferiority was not demonstrated for relapse rate; time to relapse did not differ between the groups. Severe SLE flares (renal or extrarenal) were less frequent in patients with IST continuation (5/40 vs 14/44 patients; p=0.035). Adverse events did not differ between the groups.
Conclusions: Non-inferiority of maintenance IST discontinuation after 2‒3 years was not demonstrated for renal relapse. IST discontinuation was associated with a higher risk of severe SLE flares.
Trial registration number: NCT01284725.
Keywords: Glucocorticoids; Hydroxychloroquine; Lupus Erythematosus, Systemic; Lupus Nephritis; Outcome Assessment, Health Care.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NJ-C reports receiving non-financial support from Sanofi-Genzyme and Vifor; speaking and consulting fees from Vifor and Otsuka; research grant from Fresenius Medical Care; outside the submitted work. NC-C reports research grant from Roche; outside the submitted work. LB reports speaking fees from GSK, Novartis, Biocryst, Takeda, Behring and Blueprint; research grants from Takeda, GSK, Sanofi, Biocryst, Novartis; outside the submitted work. LCh reports speaking fees from BMS and non-financial support from AstraZeneca; outside the submitted work. LCo reports speaking fees from Astellas, Chiesi, Novartis, Sandoz, Otsuka, GSK, Biotest; consultancy fees from Biotest, Hansa, Novartis; research grants from Novartis, Astellas; travel funding from Astellas, Chiesi, Novartis, Sandoz, Vifor; outside the submitted work. LD reports speaking fees from AstraZeneca and Janssen. BD reports speaking fees from Genzyme and Novonordisk; consultancy fees from Amicus; research grant from Shire; outside the submitted work. SF reports speaking fees from Asahi, Vifor and Sanofi; consultancy fees from Abyonyx Pharma; outside the submitted work. GG reports speaking fees from Genzyme, Pfizer and Novartis; non-financial support from Novartis, Amgen, Addmedicca, Lilly, Abbvie, Amicus therapeutics, Shire, Pfizer and LFB medicaments; outside the submitted work. AH reports speaking fees from Janssen and Pfizer; outside the submitted work. AK reports speaking fees from Vifor, GSK, AstraZeneca, Roche; consultancy fees from Novartis, GSK, Bohringer-Ingelheim; non-financial support from Vifor, Sanofi and Alexion; outside the submitted work. ML reports speaking fees from Chugai-Roche, Bayer, Pfizer, Leopharma; grant from Chugai-Roche; outside the submitted work. VQ reports consultancy fees from Boehringer Ingelheim and GSK; outside the submitted work. EH reports consulting fees from Johnson&Johnson, Boehringer Ingelheim, Bayer, GSK, Roche-Chugai, Sanofi-Genzyme; speaking fees from Johnson&Johnson, GSK, Roche-Chugai; research grants from CSL Behring, Johnson&Johnson, GSK, Roche-Chugai; outside the submitted work. ZA reports speaking and consultancy fees from GSK. ED reports speaking fees from GSK, Amgen, AstraZeneca; consultancy fees from GSK, AstraZeneca, Amgen; research grant from Roche; outside the submitted work.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

