Clarithromycin prevents preterm birth and neonatal mortality by dampening alarmin-induced maternal-fetal inflammation in mice
- PMID: 35725425
- PMCID: PMC9210693
- DOI: 10.1186/s12884-022-04764-2
Clarithromycin prevents preterm birth and neonatal mortality by dampening alarmin-induced maternal-fetal inflammation in mice
Abstract
Background: One of every four preterm neonates is born to a woman with sterile intra-amniotic inflammation (inflammatory process induced by alarmins); yet, this clinical condition still lacks treatment. Herein, we utilized an established murine model of sterile intra-amniotic inflammation induced by the alarmin high-mobility group box-1 (HMGB1) to evaluate whether treatment with clarithromycin prevents preterm birth and adverse neonatal outcomes by dampening maternal and fetal inflammatory responses.
Methods: Pregnant mice were intra-amniotically injected with HMGB1 under ultrasound guidance and treated with clarithromycin or vehicle control, and pregnancy and neonatal outcomes were recorded (n = 15 dams each). Additionally, amniotic fluid, placenta, uterine decidua, cervix, and fetal tissues were collected prior to preterm birth for determination of the inflammatory status (n = 7-8 dams each).
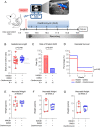
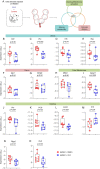
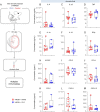
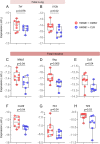
Results: Clarithromycin extended the gestational length, reduced the rate of preterm birth, and improved neonatal mortality induced by HMGB1. Clarithromycin prevented preterm birth by interfering with the common cascade of parturition as evidenced by dysregulated expression of contractility-associated proteins and inflammatory mediators in the intra-uterine tissues. Notably, clarithromycin improved neonatal survival by dampening inflammation in the placenta as well as in the fetal lung, intestine, liver, and spleen.
Conclusions: Clarithromycin prevents preterm birth and improves neonatal survival in an animal model of sterile intra-amniotic inflammation, demonstrating the potential utility of this macrolide for treating women with this clinical condition, which currently lacks a therapeutic intervention.
Keywords: Amniotic cavity; Antibiotic; Cytokine; Gene expression; HMGB1; Macrolide; Sterile intra-amniotic inflammation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

