Leveraging WHO's Global Benchmarking Tool to strengthen capacity in clinical trials oversight for public health emergencies: the GHPP VaccTrain model
- PMID: 35725614
- PMCID: PMC9207864
- DOI: 10.1186/s12992-022-00854-0
Leveraging WHO's Global Benchmarking Tool to strengthen capacity in clinical trials oversight for public health emergencies: the GHPP VaccTrain model
Abstract
Background: A stable, well-functioning and integrated national medicines regulatory system is a core component of health systems resilient against infectious disease outbreaks. In many low- and middle-income countries, however, sizable gaps exist in the emergency preparedness framework of national regulatory authorities (NRAs). RegTrain-VaccTrain is a project of Germany Ministry of Health's Global Health Protection Programme that contributes to global efforts aimed at strengthening such regulatory systems by providing technical support and advice to partner NRAs. In this study, we probed the outputs of our capacity-strengthening activities for clinical trials oversight (CTO) to take stock of progress made and examine remaining priorities in order to provide specialized technical assistance in addressing them to improve operational readiness for emergencies.
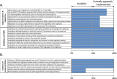
Method: Data validated from NRA self-benchmarking results in 2017 and worksheet records of November 2021 were utilized to assess the emergency preparedness capacity for CTO in three VaccTrain partner NRAs (Liberia, Sierra Leone, The Gambia) before and after interventional capacity-strengthening partnership, using specific public health emergency-related (sub-)indicators of the WHO Global Benchmarking Tool.
Results: A generally weak and vulnerable structural framework for CTO characterized the emergency preparedness capacity in all three partner NRAs at baseline, thus putting their operational readiness for public health emergencies at risk. VaccTrain's collaborative work was successful at supporting individual NRAs to develop the full spectrum of operational structures (including (draft) regulations, guidelines, and standard operating procedures) required to improve regulatory preparedness. A gap in the formal approval and implementation of developed legal documents in two of three NRAs still remains. Notwithstanding, a robust emergency framework now exists and the NRAs stand better prepared to respond to (future) locally-concerning health emergencies, during which time clinical trials activity was observed to heighten.
Conclusions: These results exemplify a north-south capacity-strengthening partnership model that effectively contributes in developing structures to enhance regulatory oversight and support expeditious product development in response to crises. They further underscore the equally critical role local/national processes play in facilitating the full implementation of developed structures.
Keywords: Capacity strengthening; Clinical trials oversight; Emergency preparedness; Evaluation; Public health emergencies; WHO GBT.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- United Nations . Transforming our world: the 2030 Agenda for sustainable development. 2015.
-
- World Health Organisation . Everybody’s Business - Strengthening Health Systems to Improve Health Outcomes: WHO’s framework for action. 2007.
-
- World Health Organisation. WHO global benchmarking tool (GBT) for evaluation of National Regulatory System of medical products. Geneva: World Health Organisation; 2021. p. 273–308. (Revision VI)
-
- Pan American Health Organisation . Considerations for regulatory oversight of clinical trials in the COVID-19 pandemic. 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

