Changing trends in clinical research literature on PubMed database from 1991 to 2020
- PMID: 35725647
- PMCID: PMC9208110
- DOI: 10.1186/s40001-022-00717-9
Changing trends in clinical research literature on PubMed database from 1991 to 2020
Abstract
Background: Clinical research publications have become the dominant source and basis of clinical evidence-based decision-making. Exploring the type and quantity of clinical research publications in the PubMed database is useful for clarifying the changing trends of clinical research development in recent years. Therefore, a longitudinal analysis of the type and quantity of clinical research publications in the PubMed database over three decades was conducted.
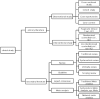
Methods: The PubMed database was searched to retrieve clinical research according to the type and year of publication from January 1, 1991 to December 31, 2020. The research types were classified as primary and secondary literature.
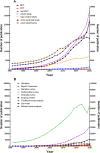
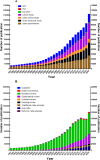
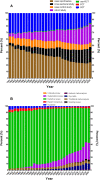
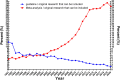
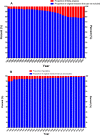
Results: A total of 1,078,404 primary literatures were retrieved and the constituent proportions were ranked from high to low as case report/series (27.54%), randomized clinical trials (RCTs) (23.62%), cohort studies (21.05%), cross-sectional studies (17.49%), case control studies (9.15%), non-RCTs (1.01%), and pragmatic clinical trials (PCTs) (0.15%). Correspondingly, 1,302,173 secondary literatures were retrieved and ranked as narrative review (70.88%), systematic review (15.02%), systematic review and meta-analyses (13.89%), traditional meta-analyses (4.48%), expert consensus (2.31%), guidelines (1.49%), scoping reviews (0.68%), net meta-analyses (0.40%), and umbrella reviews (0.04%). The average annual growth rate for the primary literature was 10.28%, and ranked from high to low as PCTs (83.68%), cohort studies (17.74%), cross-sectional studies (17.61%), non-RCTs (12.11%), case control studies (8.86%), RCTs (7.68%), case report/series (7.51%); while that for the secondary literature was 10.57%, and ranked from high to low as net meta-analyses (48.97%), umbrella reviews (47.09%), scoping reviews (41.92%), systematic reviews and meta-analyses (33.44%), systematic reviews (33.05%), traditional meta-analyses (12.49%), expert consensuses (9.22%), narrative review (8.72%), and guidelines (2.82%).
Conclusion: Both the composition and number of clinical studies changed significantly from 1991 to 2020. Based on the trend, the case report/series, case control study, and narrative review are on the decline, while cohort study, cross-sectional study, systematic reviews, and systematic review and meta-analysis literature have increased. To improve the quality of clinical evidence, we recommend RCT and cohort study give priority to access to allocated research resources in future.
Keywords: Clinical research; Data analysis; Literature summary; Longitudinal analysis; PubMed database.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources

