Automated gathering of real-world data from online patient forums can complement pharmacovigilance for rare cancers
- PMID: 35725736
- PMCID: PMC9209513
- DOI: 10.1038/s41598-022-13894-8
Automated gathering of real-world data from online patient forums can complement pharmacovigilance for rare cancers
Abstract
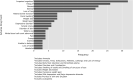
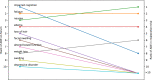
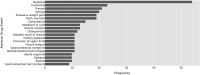
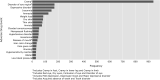
Current methods of pharmacovigilance result in severe under-reporting of adverse drug events (ADEs). Patient forums have the potential to complement current pharmacovigilance practices by providing real-time uncensored and unsolicited information. We are the first to explore the value of patient forums for rare cancers. To this end, we conduct a case study on a patient forum for Gastrointestinal Stromal Tumor patients. We have developed machine learning algorithms to automatically extract and aggregate side effects from messages on open online discussion forums. We show that patient forum data can provide suggestions for which ADEs impact quality of life the most: For many side effects the relative reporting rate differs decidedly from that of the registration trials, including for example cognitive impairment and alopecia as side effects of avapritinib. We also show that our methods can provide real-world data for long-term ADEs, such as osteoporosis and tremors for imatinib, and novel ADEs not found in registration trials, such as dry eyes and muscle cramping for imatinib. We thus posit that automated pharmacovigilance from patient forums can provide real-world data for ADEs and should be employed as input for medical hypotheses for rare cancers.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- World Health Organisation, The Safety of Medicines in Public Health Programmes: Pharmacovigilance an essential tool, 2006.
-
- Rolfes L, van Hunsel F, van der Linden L, Taxis K, van Puijenbroek E. The quality of clinical information in adverse drug reaction reports by patients and healthcare professionals: A retrospective comparative analysis. Drug Saf. 2017;40(7):607–614. doi: 10.1007/s40264-017-0530-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

