The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study
- PMID: 35725782
- PMCID: PMC9170535
- DOI: 10.1016/j.vaccine.2022.05.090
The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study
Abstract
Background: Symptoms of post-acute sequelae of COVID-19 (PASC) may improve following SARS-CoV-2 vaccination. However few prospective data that also explore the underlying biological mechanism are available. We assessed the effect of vaccination on symptomatology of participants with PASC, and compared antibody dynamics between those with and without PASC.
Methods: RECoVERED is a prospective cohort study of adult patients with mild to critical COVID-19, enrolled from illness onset. Among participants with PASC, vaccinated participants were exact-matched 1:1 on age, sex, obesity status and time since illness onset to unvaccinated participants. Between matched pairs, we compared the monthly mean numbers of symptoms over a 3-month follow-up period, and, using exact logistic regression, the proportion of participants who fully recovered from PASC. Finally, we assessed the association between PACS status and rate of decay of spike- and RBD-binding IgG titers up to 9 months after illness onset using Bayesian hierarchical linear regression.
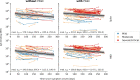
Findings: Of 349 enrolled participants, 316 (90.5%) had ≥3 months of follow-up, of whom 186 (58.9%) developed PASC. Among 36 matched pairs with PASC, the mean number of symptoms reported each month during 3 months of follow-up were comparable between vaccinated and unvaccinated groups. Odds of full recovery from PASC also did not differ between matched pairs (OR 1.57 [95%CI 0.46-5.84]) within 3 months after the matched time-point. The median half-life of spike- and RBD-binding IgG levels were, in days (95%CrI), 233 (183-324) and 181 (147-230) among participants with PASC, and 170 (125-252) and 144 (113-196) among those without PASC, respectively.
Interpretation: Our study found no strong evidence to suggest that vaccination improves symptoms of PASC. This was corroborated by comparable spike- and RBD-binding IgG waning trajectories between those with and without PASC, refuting any immunological basis for a therapeutic effect of vaccination on PASC.
Keywords: COVID-19; Long COVID; Post-acute sequelae; SARS-CoV-2; Therapeutic vaccine; Vaccination.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: A.B. received a grant from ANRS in the past 36 months and participated on the Data Safety Monitoring Board or Advisory Board for ZonMw for a study conducted by the Amsterdam University Medical Centers, location Amsterdam Medical Center. G.d.B. served as a paid member of the scientific advisory board of ExeVir in the past 36 months, and is a patent holder of INV 2020-039 both through their institution. All other authors report no potential conflicts.
Figures



References
-
- Tran V-TP, Elodie; Saldanha, Julia; Pane, Isabelle; Ravaud, Philippe. Efficacy of COVID-19 Vaccination on the Symptoms of Patients With Long COVID: A Target Trial Emulation Using Data From the ComPaRe e-Cohort in France. SSRN Pre-Print; 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

