The Impact of Patient Support Programs in Europe: A Systematic Literature Review
- PMID: 35725866
- PMCID: PMC9584873
- DOI: 10.1007/s40271-022-00582-y
The Impact of Patient Support Programs in Europe: A Systematic Literature Review
Abstract
Background and objective: Patient support programs aim to provide solutions beyond the medication itself, by enhancing treatment adherence, improving clinical outcomes, elevating patient experience, and/or increasing quality of life. As patient support programs increasingly play an important role in assisting patients, numerous observational studies and pragmatic trials designed to evaluate their impact on healthcare have been conducted in recent years. This review aims to characterize these studies.
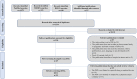
Methods: A systematic literature review, supplemented by a broad search of gray literature, was conducted following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and Cochrane recommendations. Observational studies and pragmatic trials conducted in Europe to evaluate the impact of patient support programs, published in English or Spanish between 17/03/2010 and 17/03/2020, were reviewed. Two patient support program definitions were applied starting with Ganguli et al.'s broad approach, followed by the European Medicines Agency definition, narrowed to Marketing Authorization Holders organized systems and their medicines. The quality of publications was assessed using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement 22-item checklist.
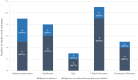
Results: Of the 49 identified studies following the Ganguli et al. definition, 20 studies met the European Medicines Agency definition and were reviewed. Patient support program impact was evaluated based on a wide range of methodologies: 70% assessed patient support program-related patient-reported outcomes, 55% reported clinical outcomes, and 25% reported economic impacts on health resources. Only 45% conducted a comparative analysis. Overall, 75% of the studies achieved their proposed objectives.
Conclusions: The heterogeneity of the observational studies reviewed reflects the complexity of patient support programs that are built ad hoc for specific diseases, treatments, and patients. Results suggest that patient support programs play a key role in promoting treatment effectiveness, clinical outcomes, and satisfaction. However, there is a need for standardizing the definition of patient support programs and the methods to evaluate their impact.
© 2022. The Author(s).
Conflict of interest statement
JAS, EA, and SDC are employees of Eli Lilly. MC, LPC, and LL work for an independent research entity that received funding to conduct the study and to write the article.
Figures
References
-
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP). Module VI: collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2). Vol. Revision 2. 2017.
-
- Pharmaceutical Industry Associations. Workshop on Patient Support and Market Research Programmes 2011. Available from: https://www.ema.europa.eu/en/documents/presentation/presentation-worksho.... [Accessed 5 May 2021].
-
- PRISMA guidelines. 2020. Available from: http://www.prisma-statement.org/ [Accessed 5 May 2021].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

