The relative abundances of yeasts attractive to Drosophila suzukii differ between fruit types and are greatest on raspberries
- PMID: 35725889
- PMCID: PMC9209449
- DOI: 10.1038/s41598-022-14275-x
The relative abundances of yeasts attractive to Drosophila suzukii differ between fruit types and are greatest on raspberries
Abstract
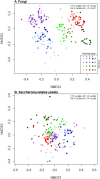
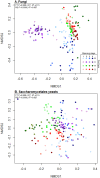
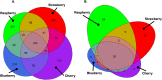
Fungal metabolic volatiles attract Drosophila suzukii which oviposits in ripening fruits, but there are few data describing the fungal microbiomes of commercial fruits susceptible to this insect pest. We tested the hypothesis that fruit type and ripening stage have a significant effect on fruit surface fungal communities using DNA metabarcoding approaches and found strong support for differences in all three fungal community biodiversity metrics analysed (numbers, types, and abundances of taxa). There was an average fivefold greater difference in fungal communities between sites with different fruit types (strawberry, cherry, raspberry, and blueberry) than across fruit developmental stages, demonstrating site and/or fruit type is the greater factor defining fungal community assemblage. The addition of a fungal internal standard (Plectosphaerella cucumerina) showed cherry had relatively static fungal populations across ripening. Raspberry had a greater prevalence of Saccharomycetales yeasts attractive to D. suzukii, including Hanseniaspora uvarum, which aligns with reports that raspberry is among the fruits with greatest susceptibility and attraction to D. suzukii. Greater knowledge of how yeast communities change during fruit maturation and between species or sites may be valuable for developing methods to manipulate fruit microbiomes for use in integrated pest management strategies to control D. suzukii.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

