ANKFN1 plays both protumorigenic and metastatic roles in hepatocellular carcinoma
- PMID: 35725908
- PMCID: PMC9287179
- DOI: 10.1038/s41388-022-02380-0
ANKFN1 plays both protumorigenic and metastatic roles in hepatocellular carcinoma
Abstract
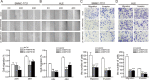
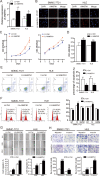
Ankyrin repeat and fibronectin type III domain containing 1 (ANKFN1) is reported to be involved in human height and developmental abnormalities, but the expression profile and molecular function of ANKFN1 in hepatocellular carcinoma (HCC) remain unknown. This study aimed to evaluate the clinical significance and biological function of ANKFN1 in HCC and investigate whether ANKFN1 can be used for differential diagnosis in HCC. Here, we showed that ANKFN1 was upregulated in 126 tumor tissues compared with adjacent nontumorous tissues in HCC patients. The upregulation of ANKFN1 in HCC was associated with cirrhosis, alpha-fetoprotein (AFP) levels and poor prognosis. Moreover, silencing ANKFN1 expression suppressed HCC cell proliferation, migration, invasion, and metastasis in vitro and subcutaneous tumorigenesis in vivo. However, ANKFN1 overexpression promoted HCC proliferation and metastasis in an orthotopic liver transplantation model and attenuated the above biological effects in HCC cells. ANKFN1 significantly affected HCC cell proliferation by inducing G1/S transition and cell apoptosis. Mechanistically, we demonstrated that ANKFN1 promoted cell proliferation, migration, and invasion via activation of the cyclin D1/Cdk4/Cdk6 pathway by stimulating the MEK1/2-ERK1/2 pathway. Moreover, ANKFN1-induced cell proliferation, migration, and invasion were partially reversed by ERK1/2 inhibitors. Taken together, our results indicate that ANKFN1 promotes HCC cell proliferation and metastasis by activating the MEK1/2-ERK1/2 signaling pathway. Our work also suggests that ANKFN1 is a potential therapeutic target for HCC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Upregulation of SNX5 predicts poor prognosis and promotes hepatocellular carcinoma progression by modulating the EGFR-ERK1/2 signaling pathway.Oncogene. 2020 Mar;39(10):2140-2155. doi: 10.1038/s41388-019-1131-9. Epub 2019 Dec 9. Oncogene. 2020. PMID: 31819169
-
RASSF7 promotes cell proliferation through activating MEK1/2-ERK1/2 signaling pathway in hepatocellular carcinoma.Cell Mol Biol (Noisy-le-grand). 2018 Apr 30;64(5):73-79. Cell Mol Biol (Noisy-le-grand). 2018. PMID: 29729697
-
miR-1307-3p promotes tumor growth and metastasis of hepatocellular carcinoma by repressing DAB2 interacting protein.Biomed Pharmacother. 2019 Sep;117:109055. doi: 10.1016/j.biopha.2019.109055. Epub 2019 Jun 5. Biomed Pharmacother. 2019. PMID: 31176165
-
The upregulation of stromal antigen 3 expression suppresses the phenotypic hallmarks of hepatocellular carcinoma through the Smad3-CDK4/CDK6-cyclin D1 and CXCR4/RhoA pathways.BMC Gastroenterol. 2022 Aug 8;22(1):378. doi: 10.1186/s12876-022-02400-z. BMC Gastroenterol. 2022. PMID: 35941537 Free PMC article.
-
MicroRNA-451: epithelial-mesenchymal transition inhibitor and prognostic biomarker of hepatocelluar carcinoma.Oncotarget. 2015 Jul 30;6(21):18613-30. doi: 10.18632/oncotarget.4317. Oncotarget. 2015. PMID: 26164082 Free PMC article.
Cited by
-
Regulating mitochondrial oxidative phosphorylation and MAPK signaling: wedelolactone as a novel therapeutic for radiation-induced thrombocytopenia.Front Pharmacol. 2025 Apr 30;16:1508215. doi: 10.3389/fphar.2025.1508215. eCollection 2025. Front Pharmacol. 2025. PMID: 40371333 Free PMC article.
-
Potential prognostic biomarker SERPINA12: implications for hepatocellular carcinoma.Clin Transl Oncol. 2025 Apr;27(4):1597-1611. doi: 10.1007/s12094-024-03689-w. Epub 2024 Sep 5. Clin Transl Oncol. 2025. PMID: 39235554 Free PMC article.
-
Plexin domain-containing 1 may be a biomarker of poor prognosis in hepatocellular carcinoma patients, may mediate immune evasion.World J Gastrointest Oncol. 2024 May 15;16(5):2091-2112. doi: 10.4251/wjgo.v16.i5.2091. World J Gastrointest Oncol. 2024. PMID: 38764846 Free PMC article.
-
Rapid Detection of PML::RARA Fusions in Acute Promyelocytic Leukemia: CRISPR/Cas9 Nanopore Sequencing with Adaptive Sampling.Biomolecules. 2024 Dec 13;14(12):1595. doi: 10.3390/biom14121595. Biomolecules. 2024. PMID: 39766302 Free PMC article.
-
Effects of in utero exposure to Δ-9-tetrahydrocannabinol on cardiac extracellular matrix expression and vascular transcriptome in rhesus macaques.Am J Physiol Heart Circ Physiol. 2024 Sep 1;327(3):H701-H714. doi: 10.1152/ajpheart.00181.2024. Epub 2024 Jul 19. Am J Physiol Heart Circ Physiol. 2024. PMID: 39028280 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

