Licogliflozin for nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a study
- PMID: 35725922
- PMCID: PMC10061496
- DOI: 10.1038/s41591-022-01861-9
Licogliflozin for nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a study
Abstract
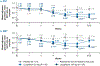
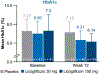
Nonalcoholic steatohepatitis (NASH) is a common chronic liver disease that may advance to fibrosis and lead to mortality; however, no pharmacotherapy is currently available. We tested the hypothesis that inhibition of both the sodium-glucose cotransporters 1 and 2 with licogliflozin would lead to improvement in NASH. A total of 107 patients with phenotypic or histologic NASH were randomized (1:2:2) to receive oral administration of either placebo (n = 21), licogliflozin 30 mg (n = 43) or 150 mg (n = 43) once daily for 12 weeks. Licogliflozin 150 mg showed a significant 32% (80% confidence interval (CI): 21-43%; P = 0.002) placebo-adjusted reduction in serum alanine aminotransferase after 12 weeks of treatment, the primary endpoint of the study. However, the 30 mg dose of licogliflozin did not meet the primary endpoint (placebo-adjusted reduction 21% (80% CI: 7-32%; P = 0.061)). Diarrhea occurred in 77% (33 of 43), 49% (21 of 43) and 43% (9 of 21) of patients treated with licogliflozin 150 mg, 30 mg and placebo, respectively, which was mostly mild in severity. No other major safety concerns were identified. Treatment with 150 mg licogliflozin led to reductions in serum alanine aminotransferase in patients with NASH. Studies of longer duration and in combination with drugs that have different mechanisms of action are needed to validate these findings and to define a role of licogliflozin as a therapeutic option for NASH. ClinicalTrials.gov identifier: NCT03205150.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures

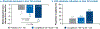
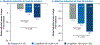


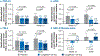



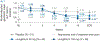

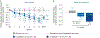
References
-
- Younossi Z et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol 15, 11–20 (2018). - PubMed
-
- Chalasani N et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018). - PubMed
-
- Williams CD et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140, 124–131 (2011). - PubMed
-
- Wong VW et al. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol. Hepatol 1, 56–67 (2016). - PubMed
-
- Younossi ZM Non-alcoholic fatty liver disease - A global public health perspective. J. Hepatol 70, 531–544 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

