SHANK3 deficiency leads to myelin defects in the central and peripheral nervous system
- PMID: 35726031
- PMCID: PMC9209365
- DOI: 10.1007/s00018-022-04400-4
SHANK3 deficiency leads to myelin defects in the central and peripheral nervous system
Abstract
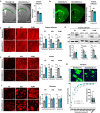
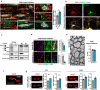
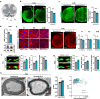
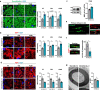
Mutations or deletions of the SHANK3 gene are causative for Phelan-McDermid syndrome (PMDS), a syndromic form of autism spectrum disorders (ASDs). We analyzed Shank3Δ11(-/-) mice and organoids from PMDS individuals to study effects on myelin. SHANK3 was found to be expressed in oligodendrocytes and Schwann cells, and MRI analysis of Shank3Δ11(-/-) mice revealed a reduced volume of the corpus callosum as seen in PMDS patients. Myelin proteins including myelin basic protein showed significant temporal and regional differences with lower levels in the CNS but increased amounts in the PNS of Shank3Δ11(-/-) animals. Node, as well as paranode, lengths were increased and ultrastructural analysis revealed region-specific alterations of the myelin sheaths. In PMDS hiPSC-derived cerebral organoids we observed an altered number and delayed maturation of myelinating cells. These findings provide evidence that, in addition to a synaptic deregulation, impairment of myelin might profoundly contribute to the clinical manifestation of SHANK3 deficiency.
Keywords: ASD; Myelin; SHANK3; hiPSCs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Increased rates of cerebral protein synthesis in Shank3 knockout mice: Implications for a link between synaptic protein deficit and dysregulated protein synthesis in autism spectrum disorder/intellectual disability.Neurobiol Dis. 2021 Jan;148:105213. doi: 10.1016/j.nbd.2020.105213. Epub 2020 Dec 1. Neurobiol Dis. 2021. PMID: 33276083
-
Disrupted extracellular matrix and cell cycle genes in autism-associated Shank3 deficiency are targeted by lithium.Mol Psychiatry. 2024 Mar;29(3):704-717. doi: 10.1038/s41380-023-02362-y. Epub 2023 Dec 20. Mol Psychiatry. 2024. PMID: 38123724 Free PMC article.
-
Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice.Mol Psychiatry. 2017 May;22(5):689-702. doi: 10.1038/mp.2016.30. Epub 2016 Mar 29. Mol Psychiatry. 2017. PMID: 27021819 Free PMC article.
-
Comparison of SHANK3 deficiency in animal models: phenotypes, treatment strategies, and translational implications.J Neurodev Disord. 2021 Nov 16;13(1):55. doi: 10.1186/s11689-021-09397-8. J Neurodev Disord. 2021. PMID: 34784886 Free PMC article. Review.
-
Zinc deficiency and supplementation in autism spectrum disorder and Phelan-McDermid syndrome.J Neurosci Res. 2022 Apr;100(4):970-978. doi: 10.1002/jnr.25019. Epub 2022 Feb 3. J Neurosci Res. 2022. PMID: 35114017 Review.
Cited by
-
Multisensory gamma stimulation mitigates the effects of demyelination induced by cuprizone in male mice.Nat Commun. 2024 Aug 8;15(1):6744. doi: 10.1038/s41467-024-51003-7. Nat Commun. 2024. PMID: 39112447 Free PMC article.
-
Development of sex- and genotype-specific behavioral phenotypes in a Shank3 mouse model for neurodevelopmental disorders.Front Behav Neurosci. 2023 Jan 9;16:1051175. doi: 10.3389/fnbeh.2022.1051175. eCollection 2022. Front Behav Neurosci. 2023. PMID: 36699652 Free PMC article.
-
Human pluripotent stem cell (hPSC) and organoid models of autism: opportunities and limitations.Transl Psychiatry. 2023 Jun 21;13(1):217. doi: 10.1038/s41398-023-02510-6. Transl Psychiatry. 2023. PMID: 37344450 Free PMC article. Review.
-
Age-dependent cortical overconnectivity in Shank3 mice is reversed by anesthesia.Transl Psychiatry. 2025 Apr 19;15(1):154. doi: 10.1038/s41398-025-03377-5. Transl Psychiatry. 2025. PMID: 40253406 Free PMC article.
-
SCN2A-linked myelination deficits and synaptic plasticity alterations drive auditory processing disorders in ASD.Res Sq [Preprint]. 2024 Aug 28:rs.3.rs-4925935. doi: 10.21203/rs.3.rs-4925935/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Aug 2;16(1):7109. doi: 10.1038/s41467-025-62494-3. PMID: 39257993 Free PMC article. Updated. Preprint.
References
-
- DSM-V DDMNSYPICfDCP, Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. Morb Mortal Wkly Rep Recomm Rep. 2009;58(10):1–20. - PubMed
-
- Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease C, Prevention. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and mortality weekly report Surveillance summaries. 2014;63(2):1–21. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

