Rapid biosensor development using plant hormone receptors as reprogrammable scaffolds
- PMID: 35726092
- PMCID: PMC9750858
- DOI: 10.1038/s41587-022-01364-5
Rapid biosensor development using plant hormone receptors as reprogrammable scaffolds
Abstract
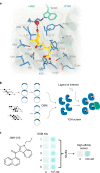
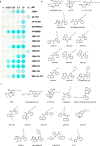
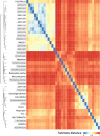
A general method to generate biosensors for user-defined molecules could provide detection tools for a wide range of biological applications. Here, we describe an approach for the rapid engineering of biosensors using PYR1 (Pyrabactin Resistance 1), a plant abscisic acid (ABA) receptor with a malleable ligand-binding pocket and a requirement for ligand-induced heterodimerization, which facilitates the construction of sense-response functions. We applied this platform to evolve 21 sensors with nanomolar to micromolar sensitivities for a range of small molecules, including structurally diverse natural and synthetic cannabinoids and several organophosphates. X-ray crystallography analysis revealed the mechanistic basis for new ligand recognition by an evolved cannabinoid receptor. We demonstrate that PYR1-derived receptors are readily ported to various ligand-responsive outputs, including enzyme-linked immunosorbent assay (ELISA)-like assays, luminescence by protein-fragment complementation and transcriptional circuits, all with picomolar to nanomolar sensitivity. PYR1 provides a scaffold for rapidly evolving new biosensors for diverse sense-response applications.
© 2022. The Author(s).
Conflict of interest statement
P.J.S., M.B., T.A.W, S.R.C., I.W. and J.B. have filed a provisional patent entitled REAGENTS AND SYSTEMS FOR GENERATING BIOSENSORS (US9738902B2; WO2011139798A2) covering the research in the present work.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

