Albumin Glycation Affects the Delivery of C-Peptide to the Red Blood Cells
- PMID: 35726250
- PMCID: PMC9204818
- DOI: 10.1021/acsmeasuresciau.2c00001
Albumin Glycation Affects the Delivery of C-Peptide to the Red Blood Cells
Abstract
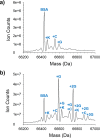
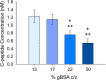
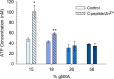
Serum albumin is a prominent plasma protein that becomes modified in hyperglycemic conditions. In a process known as glycation, these modifications can change the structure and function of proteins, which decrease ligand binding capabilities and alter the bioavailability of ligands. C-peptide is a molecule that binds to the red blood cell (RBC) and stimulates the release of adenosine triphosphate (ATP), which is known to participate in the regulation of blood flow. C-peptide binding to the RBC only occurs in the presence of albumin, and downstream signaling cascades only occur when the albumin and C-peptide complex contains Zn2+. Here, we measure the binding of glycated bovine serum albumin (gBSA) to the RBC in conditions with or without C-peptide and Zn2+. Key to these studies is the analytical sample preparation involving separation of BSA fractions with boronate affinity chromatography and characterization of the varying glycation levels with mass spectrometry. Results from this study show an increase in binding for higher % glycation of gBSA to the RBCs, but a decrease in ability to deliver C-peptide (0.75 ± 0.11 nM for 22% gBSA) compared to samples with less glycation (1.22 ± 0.16 nM for 13% gBSA). A similar trend was measured for Zn2+ delivery to the RBC as a function of glycation percentage. When 15% gBSA or 18% gBSA was combined with C-peptide/Zn2+, the derived ATP release from the RBCs significantly increased to 113% or 36%, respectively. However, 26% gBSA with C-peptide/Zn2+ had no significant increase in ATP release from RBCs. These results indicate that glycation of BSA interferes in C-peptide and Zn2+ binding to the RBC and subsequent RBC ATP release, which may have implications in C-peptide therapy for people with type 1 diabetes.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): DMS has a financial interest in LifeBlood, which has a diagnostic based on C-peptide binding to cells from people with Multiple Sclerosis.
Figures



References
-
- Center for Disease Control and Prevention . Underlying Cause of Death 1999–2016, 2016.
-
- Center for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2020.
Grants and funding
LinkOut - more resources
Full Text Sources
