Journey on the Radical SAM Road as an Accidental Pilgrim
- PMID: 35726327
- PMCID: PMC9204691
- DOI: 10.1021/acsbiomedchemau.1c00059
Journey on the Radical SAM Road as an Accidental Pilgrim
Abstract
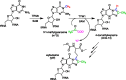
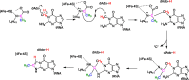
Radical S-adenosyl-l-methionine (SAM) enzymes catalyze a diverse group of complex transformations in all aspects of cellular physiology. These metalloenzymes bind SAM to a 4Fe-4S cluster and reductively cleave SAM to generate a 5'-deoxyadenosyl radical, which generally initiates the catalytic cycle by catalyzing a H atom to activate the substrate for subsequent chemistry. This perspective will focus on our discovery of several members of this superfamily of enzymes, with a particular emphasis on the current state of the field, challenges, and outlook.
© 2022 The Author. Published by American Chemical Society.
Conflict of interest statement
The author declares no competing financial interest.
Figures

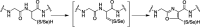
Similar articles
-
Radical SAM enzymes in methylation and methylthiolation.Metallomics. 2012 Nov;4(11):1149-54. doi: 10.1039/c2mt20136d. Epub 2012 Sep 19. Metallomics. 2012. PMID: 22992596 Free PMC article. Review.
-
Structure and Catalytic Mechanism of Radical SAM Methylases.Life (Basel). 2022 Oct 28;12(11):1732. doi: 10.3390/life12111732. Life (Basel). 2022. PMID: 36362886 Free PMC article. Review.
-
Expanding Radical SAM Chemistry by Using Radical Addition Reactions and SAM Analogues.Angew Chem Int Ed Engl. 2016 Sep 19;55(39):11845-8. doi: 10.1002/anie.201605917. Epub 2016 Aug 30. Angew Chem Int Ed Engl. 2016. PMID: 27573794
-
Intermolecular electron transfer in radical SAM enzymes as a new paradigm for reductive activation.J Biol Chem. 2023 Sep;299(9):105058. doi: 10.1016/j.jbc.2023.105058. Epub 2023 Jul 17. J Biol Chem. 2023. PMID: 37460016 Free PMC article.
-
Trapping and Electron Paramagnetic Resonance Characterization of the 5'dAdo• Radical in a Radical S-Adenosyl Methionine Enzyme Reaction with a Non-Native Substrate.ACS Cent Sci. 2019 Nov 27;5(11):1777-1785. doi: 10.1021/acscentsci.9b00706. Epub 2019 Sep 25. ACS Cent Sci. 2019. PMID: 31807679 Free PMC article.
Cited by
-
In Vitro Demonstration of Human Lipoyl Synthase Catalytic Activity in the Presence of NFU1.ACS Bio Med Chem Au. 2022 Oct 19;2(5):456-468. doi: 10.1021/acsbiomedchemau.2c00020. Epub 2022 Jun 13. ACS Bio Med Chem Au. 2022. PMID: 36281303 Free PMC article.
-
Twenty Years of Radical SAM! The Genesis of the Superfamily.ACS Bio Med Chem Au. 2022 Dec 5;2(6):538-547. doi: 10.1021/acsbiomedchemau.2c00078. eCollection 2022 Dec 21. ACS Bio Med Chem Au. 2022. PMID: 37101427 Free PMC article. No abstract available.
-
Not all 5'-deoxyadenosines are created equal: Tracing the provenance of 5'-deoxyadenosine formed by the radical S-adenosyl-L-methionine enzyme 7-carboxy-7-deazaguanine synthase.J Biol Chem. 2025 Apr;301(4):108347. doi: 10.1016/j.jbc.2025.108347. Epub 2025 Feb 25. J Biol Chem. 2025. PMID: 40015645 Free PMC article.
-
Mechanistic Insights from the Crystal Structure and Computational Analysis of the Radical SAM Deaminase DesII.Adv Sci (Weinh). 2024 Sep;11(33):e2403494. doi: 10.1002/advs.202403494. Epub 2024 Jun 28. Adv Sci (Weinh). 2024. PMID: 38943270 Free PMC article.
References
-
- Sofia H. J.; Chen G.; Hetzler B. G.; Reyes-Spindola J. F.; Miller N. E. Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 2001, 29, 1097–1106. 10.1093/nar/29.5.1097. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

