Journey on the Radical SAM Road as an Accidental Pilgrim
- PMID: 35726327
- PMCID: PMC9204691
- DOI: 10.1021/acsbiomedchemau.1c00059
Journey on the Radical SAM Road as an Accidental Pilgrim
Abstract
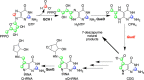
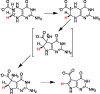
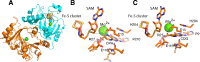
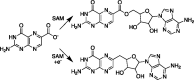
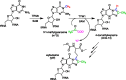
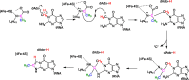
Radical S-adenosyl-l-methionine (SAM) enzymes catalyze a diverse group of complex transformations in all aspects of cellular physiology. These metalloenzymes bind SAM to a 4Fe-4S cluster and reductively cleave SAM to generate a 5'-deoxyadenosyl radical, which generally initiates the catalytic cycle by catalyzing a H atom to activate the substrate for subsequent chemistry. This perspective will focus on our discovery of several members of this superfamily of enzymes, with a particular emphasis on the current state of the field, challenges, and outlook.
© 2022 The Author. Published by American Chemical Society.
Conflict of interest statement
The author declares no competing financial interest.
Figures

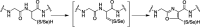
References
-
- Sofia H. J.; Chen G.; Hetzler B. G.; Reyes-Spindola J. F.; Miller N. E. Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 2001, 29, 1097–1106. 10.1093/nar/29.5.1097. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

