Decoding the transcriptome of denervated muscle at single-nucleus resolution
- PMID: 35726356
- PMCID: PMC9398230
- DOI: 10.1002/jcsm.13023
Decoding the transcriptome of denervated muscle at single-nucleus resolution
Abstract
Background: Skeletal muscle exhibits remarkable plasticity under both physiological and pathological conditions. One major manifestation of this plasticity is muscle atrophy that is an adaptive response to catabolic stimuli. Because the heterogeneous transcriptome responses to catabolism in different types of muscle cells are not fully characterized, we applied single-nucleus RNA sequencing (snRNA-seq) to unveil muscle atrophy related transcriptional changes at single nucleus resolution.
Methods: Using a sciatic denervation mouse model of muscle atrophy, snRNA-seq was performed to generate single-nucleus transcriptional profiles of the gastrocnemius muscle from normal and denervated mice. Various bioinformatics analyses, including unsupervised clustering, functional enrichment analysis, trajectory analysis, regulon inference, metabolic signature characterization and cell-cell communication prediction, were applied to illustrate the transcriptome changes of the individual cell types.
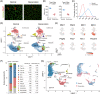
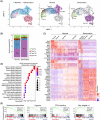
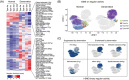
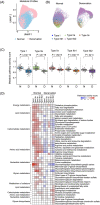
Results: A total of 29 539 muscle nuclei (normal vs. denervation: 15 739 vs. 13 800) were classified into 13 nuclear types according to the known cell markers. Among these, the type IIb myonuclei were further divided into two subgroups, which we designated as type IIb1 and type IIb2 myonuclei. In response to denervation, the proportion of type IIb2 myonuclei increased sharply (78.12% vs. 38.45%, P < 0.05). Concomitantly, trajectory analysis revealed that denervated type IIb2 myonuclei clearly deviated away from the normal type IIb2 myonuclei, indicating that this subgroup underwent robust transcriptional reprogramming upon denervation. Signature genes in denervated type IIb2 myonuclei included Runx1, Gadd45a, Igfn1, Robo2, Dlg2, and Sh3d19 (P < 0.001). The gene regulatory network analysis captured a group of atrophy-related regulons (Foxo3, Runx1, Elk4, and Bhlhe40) whose activities were enhanced (P < 0.01), especially in the type IIb2 myonuclei. The metabolic landscape in the myonuclei showed that most of the metabolic pathways were down-regulated by denervation (P < 0.001), while some of the metabolic signalling, such as glutathione metabolism, was specifically activated in the denervated type IIb2 myonulei. We also investigated the transcriptomic alterations in the type I myofibres, muscle stem cells, fibro-adipogenic progenitors, macrophages, endothelial cells and pericytes and characterized their signature responses to denervation. By predicting the cell-cell interactions, we observed that the communications between myofibres and muscle resident cells were diminished by denervation.
Conclusions: Our results define the myonuclear transition, metabolic remodelling, and gene regulation networks reprogramming associated with denervation-induced muscle atrophy and illustrate the molecular basis of the heterogeneity and plasticity of muscle cells in response to catabolism. These results provide a useful resource for exploring the molecular mechanism of muscle atrophy.
Keywords: Denervation; Muscle atrophy; Muscle metabolism; Skeletal muscle; snRNA-seq.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
All authors declare no conflicting financial interests. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the
Figures




References
-
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 2011;91:1447–1531. - PubMed
-
- Nicoletti C, Wei X, Etxaniz U, Proietti D, Madaro L, Puri PL. scRNA‐seq‐based analysis of skeletal muscle response to denervation reveals selective activation of muscle‐resident glial cells and fibroblasts. bioRxiv. 2020; 2020.12.29.424762.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

