Cost-Effectiveness of Catheter Ablation Versus Antiarrhythmic Drug Therapy in Atrial Fibrillation: The CABANA Randomized Clinical Trial
- PMID: 35726631
- PMCID: PMC9378541
- DOI: 10.1161/CIRCULATIONAHA.122.058575
Cost-Effectiveness of Catheter Ablation Versus Antiarrhythmic Drug Therapy in Atrial Fibrillation: The CABANA Randomized Clinical Trial
Abstract
Background: In the CABANA trial (Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation), catheter ablation did not significantly reduce the primary end point of death, disabling stroke, serious bleeding, or cardiac arrest compared with drug therapy by intention-to-treat, but did improve the quality of life and freedom from atrial fibrillation recurrence. In the heart failure subgroup, ablation improved both survival and quality of life. Cost-effectiveness was a prespecified CABANA secondary end point.
Methods: Medical resource use data were collected for all CABANA patients (N=2204). Costs for hospital-based care were assigned using prospectively collected bills from US patients (n=1171); physician and medication costs were assigned using the Medicare Fee Schedule and National Average Drug Acquisition Costs, respectively. Extrapolated life expectancies were estimated using age-based survival models. Quality-of-life adjustments were based on EQ-5D-based utilities measured during the trial. The primary outcome was the incremental cost-effectiveness ratio, comparing ablation with drug therapy on the basis of intention-to-treat, and assessed from the US health care sector perspective.
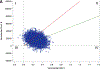
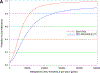
Results: Costs in the first 3 months averaged $20 794±SD 1069 higher with ablation compared with drug therapy. The cumulative within-trial 5-year cost difference was $19 245 (95% CI, $11 360-$27 170) and the lifetime mean cost difference was $15 516 (95% CI, -$2963 to $35,512) higher with ablation than with drug therapy. The drug therapy arm accrued an average of 12.5 life-years (LYs) and 10.7 quality-adjusted life-years (QALYs). For the ablation arm, the corresponding estimates were 12.6 LYs and 11.0 QALYs. The incremental cost-effectiveness ratio was $57 893 per QALY gained, with 75% of bootstrap replications yielding an incremental cost-effectiveness ratio <$100 000 per QALY gained. With no quality-of-life/utility adjustments, the incremental cost-effectiveness ratio was $183 318 per LY gained.
Conclusions: Catheter ablation of atrial fibrillation was economically attractive compared with drug therapy in the CABANA Trial overall at present benchmarks for health care value in the United States on the basis of projected incremental QALYs but not LYs alone.
Keywords: anti-arrhythmia agents; atrial fibrillation; catheter ablation; drug therapy; health economics; pulmonary vein.
Conflict of interest statement
DISCLOSURES
Dr. Chew is supported by a Canadian Institutes of Health Research Banting Fellowship and an Arthur JE Child Cardiology Fellowship outside the submitted work.
Dr. Cowper reports research grant support from Mayo Clinic and NIH/NHLBI for the submitted work, and research grant support from Merck, Eli Lilly, Novartis, AstraZeneca, Bristol Myers Squibb, AGA Medical, Tenax Therapeutics, GE Healthcare and Gilead outside the submitted work.
Dr. Piccini is supported by R01HL128595 from the National Heart, Lung and Blood Institute and receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, NIH, and Philips; consulting from Abbott, Abbvie, Ablacon, Altathera, ARCA Biopharma, Biotronik, Boston Scientific, Bristol Myers Squibb, LivaNova, Medtronic, Milestone, ElectroPhysiology Frontiers, Pfizer, Sanofi, Philips, and Up-to-Date outside the submitted work.
Dr. Poole reports research grants paid directly to the University of Washington from Biotonik, Kestra Medical and AtriCure.
Ms. Monahan reports grants from NIH/NHLBI, St. Jude Foundation and Corporation, Biosense Webster, Inc., Medtronic, Inc, and Boston Scientific Corp, during the conduct of the study; consulting without compensation from Biosense Webster, Inc; and personal fees from Thermedical outside the submitted work.
Dr. Al-Khalidi reports grants from the NIH/NHLBI and Mayo Clinic during the conduct of the study.
Dr. Bahnson reports grants from the NIH/NHLBI and Mayo Clinic during the conduct of the study; grants from St. Jude Medical, Inc, Abbott Medical, Biosense Webster Inc, Johnson & Johnson, NIH, and Boston Scientific Corp.
Dr. Lee reports grants from the NIH/NHLBI and Mayo Clinic, as well as Data Safety and Monitoring Board service on studies funded by Astra-Zeneca, Medtronic, Merck, Amgen, and the Cardiovascular Research Foundation during the conduct of the study.
Dr. Packer in the past 12 months has provided consulting services for Abbott, AtriFix, Biosense Webster, Inc., Cardio Syntax, EBAmed, Johnson & Johnson, MediaSphere Medical, LLC, MedLumics, Medtronic, NeuCures, St. Jude Medical, Siemens, Spectrum Dynamics, Centrix, and Thermedical. Dr. Packer received no personal compensation for these consulting activities, unless noted. Dr. Packer receives research funding from the Abbott, Biosense Webster, Boston Scientific/EPT, CardioInsight, EBAmed, Medtronic, Inc, Siemens, St. Jude Medical, Inc, Thermedical, Inc., NIH, Robertson Foundation, Vital Project Funds, Inc., Mr. and Mrs. J. Michael Cook/Fund. Mayo Clinic and Dr. Packer have a financial interest in Analyze-AVW technology that may have been used to analyze some of the heart images in this research. In accordance with the Bayh-Dole Act, this technology has been licensed to commercial entities, and both Mayo Clinic and Dr. Packer have received royalties greater than $10,000, the federal threshold for significant financial interest. In addition, Mayo Clinic holds an equity position in the company to which the AVW technology has been licensed. Dr. Packer and Mayo Clinic jointly have equity in a privately held company, EBAmed. Royalties from Wiley & Sons, Oxford, and St. Jude Medical.
Dr. Mark reports grants from NIH/NHLBI and Mayo Clinic during the conduct of the study; grants from Merck and HeartFlow; and personal fees from Novartis outside the submitted work.
The remaining authors report no conflicts.
Figures


References
-
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB and Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. - PubMed
-
- Dorian P, Paquette M, Newman D, Green M, Connolly SJ, Talajic M and Roy D. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J 2002;143:984–990. - PubMed
-
- Kim MH, Johnston SS, Chu BC, Dalal MR and Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–320. - PubMed
-
- Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019;321:1275–1285. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

