Bacterial, Phytoplankton, and Viral Distributions and Their Biogeochemical Contexts in Meromictic Lake Cadagno Offer Insights into the Proterozoic Ocean Microbial Loop
- PMID: 35726916
- PMCID: PMC9426590
- DOI: 10.1128/mbio.00052-22
Bacterial, Phytoplankton, and Viral Distributions and Their Biogeochemical Contexts in Meromictic Lake Cadagno Offer Insights into the Proterozoic Ocean Microbial Loop
Abstract
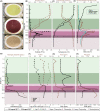
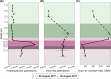
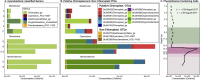
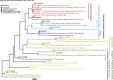
Lake Cadagno, a permanently stratified high-alpine lake with a persistent microbial bloom in its chemocline, has long been considered a model for the low-oxygen, high-sulfide Proterozoic ocean. Although the lake has been studied for over 25 years, the absence of concerted study of the bacteria, phytoplankton, and viruses, together with primary and secondary production, has hindered a comprehensive understanding of its microbial food web. Here, the identities, abundances, and productivity of microbes were evaluated in the context of Lake Cadagno biogeochemistry. Photosynthetic pigments together with 16S rRNA gene phylogenies suggest the prominence of eukaryotic phytoplankton chloroplasts, primarily chlorophytes. Chloroplasts closely related to those of high-alpine-adapted Ankyra judayi persisted with oxygen in the mixolimnion, where photosynthetic efficiency was high, while chloroplasts of Closteriopsis-related chlorophytes peaked in the chemocline and monimolimnion. The anoxygenic phototrophic sulfur bacterium Chromatium dominated the chemocline along with Lentimicrobium, a genus of known fermenters. Secondary production peaked in the chemocline, which suggested that anoxygenic primary producers depended on heterotrophic nutrient remineralization. The virus-to-microbe ratio peaked with phytoplankton abundances in the mixolimnion and were at a minimum where Chromatium abundance was highest, trends that suggest that viruses may play a role in the modulation of primary production. Through the combined analysis of bacterial, eukaryotic, viral, and biogeochemical spatial dynamics, we provide a comprehensive synthesis of the Lake Cadagno microbial loop. This study offers a new ecological perspective on how biological and geochemical connections may have occurred in the chemocline of the Proterozoic ocean, where eukaryotic microbial life is thought to have evolved. IMPORTANCE As a window into the past, this study offers insights into the potential role that microbial guilds may have played in the production and recycling of organic matter in ancient Proterozoic ocean chemoclines. The new observations described here suggest that chloroplasts of eukaryotic algae were persistent in the low-oxygen upper chemocline along with the purple and green sulfur bacteria known to dominate the lower half of the chemocline. This study provides the first insights into Lake Cadagno's viral ecology. High viral abundances suggested that viruses may be essential components of the chemocline, where their activity may result in the release and recycling of organic matter. The integration of diverse geochemical and biological data types provides a framework that lays the foundation to quantitatively resolve the processes performed by the discrete populations that comprise the microbial loop in this early anoxic ocean analogue.
Keywords: Lake Cadagno; Proterozoic; ancient ocean; meromictic; microbial loop; viral ecology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Canfield DE. 1998. A new model for Proterozoic ocean chemistry. Nature 396:450–453. doi:10.1038/24839. - DOI
-
- Gulati RD, Zadereev ES, Degermendzhi AG (ed). 2017. Ecology of meromictic lakes. Springer International Publishing, Cham, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

