Efficacy and Safety of Varenicline for Smoking Cessation in Patients With Type 2 Diabetes: A Randomized Clinical Trial
- PMID: 35727580
- PMCID: PMC9214580
- DOI: 10.1001/jamanetworkopen.2022.17709
Efficacy and Safety of Varenicline for Smoking Cessation in Patients With Type 2 Diabetes: A Randomized Clinical Trial
Abstract
Importance: Evidence of effective smoking cessation interventions in patients with diabetes is limited. The unique behavioral and metabolic characteristics of smokers with type 2 diabetes warrants a randomized clinical trial of the smoking cessation drug varenicline.
Objective: To evaluate the efficacy and safety of varenicline in patients with type 2 diabetes with an intention to quit smoking.
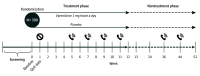
Design, setting, and participants: This multicenter, double-blind, placebo-controlled randomized clinical trial recruited patients from 6 outpatient clinics in 5 hospitals in Catania, Italy. Patients with type 2 diabetes, who were smoking at least 10 cigarettes a day, and who intended to quit smoking were screened for eligibility. Eligible patients were randomized to either varenicline or placebo treatment. The trial consisted of a 12-week treatment phase followed by a 40-week follow-up, nontreatment phase. Intention-to-treat data analysis was performed from December 2020 to April 2021.
Interventions: Varenicline, 1 mg, twice daily or matched placebo administered for 12 weeks. Patients in both treatment groups also received smoking cessation counseling.
Main outcomes and measures: The primary efficacy end point of the study was the continuous abstinence rate (CAR) at weeks 9 to 24. Secondary efficacy end points were the CAR at weeks 9 to 12 and weeks 9 to 52 as well as 7-day point prevalence of abstinence at weeks 12, 24, and 52.
Results: A total of 300 patients (mean [SD] age, 57.4 [0.8] years; 117 men [78.0%] in varenicline group and 119 men [79.3%] in placebo group) were randomized to receive varenicline (n = 150) or placebo (n = 150). The CAR at weeks 9 to 24 was significantly higher for the varenicline than placebo group (24.0% vs 6.0%; odds ratio [OR], 4.95; 95% CI, 2.29-10.70; P < .001). The CARs at weeks 9 to 12 (31.3% vs 7.3%; OR, 5.77; 95% CI, 2.85-11.66; P < .001) and weeks 9 to 52 (18.7% vs 5.3%; OR, 4.07; 95% CI, 1.79-9.27; P < .001) as well as the 7-day point prevalence of abstinence at weeks 12, 24, and 52 were also significantly higher for the varenicline vs placebo group. The most frequent adverse events occurring in the varenicline group compared with the placebo group were nausea (41 [27.3%] vs 17 [11.4%]), insomnia (29 [19.4%] vs 19 [12.7%]), abnormal dreams (19 [12.7%] vs 5 [3.4%]), anxiety (17 [11.4%] vs 11 [7.3%]), and irritability (14 [9.4%] vs 8 [5.4%]). Serious adverse events were infrequent in both groups and not treatment-related.
Conclusions and relevance: Results of this trial showed that inclusion of varenicline in a smoking cessation program is efficacious in achieving long-term abstinence without serious adverse events. Varenicline should be routinely used in diabetes education programs to help patients with type 2 diabetes stop smoking.
Trial registration: ClinicalTrials.gov Identifier: NCT01387425.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

