Evaluation of the Growth Assessment Protocol (GAP) for antenatal detection of small for gestational age: The DESiGN cluster randomised trial
- PMID: 35727800
- PMCID: PMC9212153
- DOI: 10.1371/journal.pmed.1004004
Evaluation of the Growth Assessment Protocol (GAP) for antenatal detection of small for gestational age: The DESiGN cluster randomised trial
Abstract
Background: Antenatal detection and management of small for gestational age (SGA) is a strategy to reduce stillbirth. Large observational studies provide conflicting results on the effect of the Growth Assessment Protocol (GAP) in relation to detection of SGA and reduction of stillbirth; to the best of our knowledge, there are no reported randomised control trials. Our aim was to determine if GAP improves antenatal detection of SGA compared to standard care.
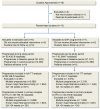
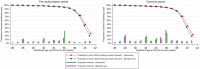
Methods and findings: This was a pragmatic, superiority, 2-arm, parallel group, open, cluster randomised control trial. Maternity units in England were eligible to participate in the study, except if they had already implemented GAP. All women who gave birth in participating clusters (maternity units) during the year prior to randomisation and during the trial (November 2016 to February 2019) were included. Multiple pregnancies, fetal abnormalities or births before 24+1 weeks were excluded. Clusters were randomised to immediate implementation of GAP, an antenatal care package aimed at improving detection of SGA as a means to reduce the rate of stillbirth, or to standard care. Randomisation by random permutation was stratified by time of study inclusion and cluster size. Data were obtained from hospital electronic records for 12 months prerandomisation, the washout period (interval between randomisation and data collection of outcomes), and the outcome period (last 6 months of the study). The primary outcome was ultrasound detection of SGA (estimated fetal weight <10th centile using customised centiles (intervention) or Hadlock centiles (standard care)) confirmed at birth (birthweight <10th centile by both customised and population centiles). Secondary outcomes were maternal and neonatal outcomes, including induction of labour, gestational age at delivery, mode of birth, neonatal morbidity, and stillbirth/perinatal mortality. A 2-stage cluster-summary statistical approach calculated the absolute difference (intervention minus standard care arm) adjusted using the prerandomisation estimate, maternal age, ethnicity, parity, and randomisation strata. Intervention arm clusters that made no attempt to implement GAP were excluded in modified intention to treat (mITT) analysis; full ITT was also reported. Process evaluation assessed implementation fidelity, reach, dose, acceptability, and feasibility. Seven clusters were randomised to GAP and 6 to standard care. Following exclusions, there were 11,096 births exposed to the intervention (5 clusters) and 13,810 exposed to standard care (6 clusters) during the outcome period (mITT analysis). Age, height, and weight were broadly similar between arms, but there were fewer women: of white ethnicity (56.2% versus 62.7%), and in the least deprived quintile of the Index of Multiple Deprivation (7.5% versus 16.5%) in the intervention arm during the outcome period. Antenatal detection of SGA was 25.9% in the intervention and 27.7% in the standard care arm (adjusted difference 2.2%, 95% confidence interval (CI) -6.4% to 10.7%; p = 0.62). Findings were consistent in full ITT analysis. Fidelity and dose of GAP implementation were variable, while a high proportion (88.7%) of women were reached. Use of routinely collected data is both a strength (cost-efficient) and a limitation (occurrence of missing data); the modest number of clusters limits our ability to study small effect sizes.
Conclusions: In this study, we observed no effect of GAP on antenatal detection of SGA compared to standard care. Given variable implementation observed, future studies should incorporate standardised implementation outcomes such as those reported here to determine generalisability of our findings.
Trial registration: This trial is registered with the ISRCTN registry, ISRCTN67698474.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: NM reports personal fees from Takeda, personal fees from RSM Consulting, personal fees from Novartis, outside the submitted work. BT is the Clinical Director of the Tommy’s National Centre for Maternity Improvement based at the Royal College of Obstetrics and Gynaecology (RCOG); the Centre’s objective is to translate the latest evidence into clinical practice in the UK. DAL has received support from Medtronic Ltd and Roche Diagnostics for research unrelated to that presented here. LP is clinical advisor [and from Sept 2021 deputy clinical director] for Healthcare Safety Investigation Branch maternity investigation programme, President of the British Intrapartum Care Society (BICS), invited member of some RCOG working groups and co-opted member of the British Maternal and Fetal Medicine Society (BMFMS) committee; she also received support from Pharmacosmos for clinical consultancy in work unrelated to that presented here.
Figures
References
-
- UNICEF, WHO. Every Newborn: an action plan to end preventable deaths. World Health Organization, Geneva: 2014; 2014 [cited 2020 Jan 10]. https://www.who.int/maternal_child_adolescent/newborns/every-newborn/en/
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

