Thromboxane-Prostanoid Receptor Signaling Drives Persistent Fibroblast Activation in Pulmonary Fibrosis
- PMID: 35728047
- PMCID: PMC9716913
- DOI: 10.1164/rccm.202106-1503OC
Thromboxane-Prostanoid Receptor Signaling Drives Persistent Fibroblast Activation in Pulmonary Fibrosis
Abstract
Rationale: Although persistent fibroblast activation is a hallmark of idiopathic pulmonary fibrosis (IPF), mechanisms regulating persistent fibroblast activation in the lungs have not been fully elucidated. Objectives: On the basis of our observation that lung fibroblasts express TBXA2R (thromboxane-prostanoid receptor) during fibrosis, we investigated the role of TBXA2R signaling in fibrotic remodeling. Methods: We identified TBXA2R expression in lungs of patients with IPF and mice and studied primary mouse and human lung fibroblasts to determine the impact of TBXA2R signaling on fibroblast activation. We used TBXA2R-deficient mice and small-molecule inhibitors to investigate TBXA2R signaling in preclinical lung fibrosis models. Measurements and Main Results: TBXA2R expression was upregulated in fibroblasts in the lungs of patients with IPF and in mouse lungs during experimental lung fibrosis. Genetic deletion of TBXA2R, but not inhibition of thromboxane synthase, protected mice from bleomycin-induced lung fibrosis, thereby suggesting that an alternative ligand activates profibrotic TBXA2R signaling. In contrast to thromboxane, F2-isoprostanes, which are nonenzymatic products of arachidonic acid induced by reactive oxygen species, were persistently elevated during fibrosis. F2-isoprostanes induced TBXA2R signaling in fibroblasts and mediated a myofibroblast activation profile due, at least in part, to potentiation of TGF-β (transforming growth factor-β) signaling. In vivo treatment with the TBXA2R antagonist ifetroban reduced profibrotic signaling in the lungs, protected mice from lung fibrosis in three preclinical models (bleomycin, Hermansky-Pudlak mice, and radiation-induced fibrosis), and markedly enhanced fibrotic resolution after bleomycin treatment. Conclusions: TBXA2R links oxidative stress to fibroblast activation during lung fibrosis. TBXA2R antagonists could have utility in treating pulmonary fibrosis.
Keywords: Hermansky-Pudlak syndrome; IPF; oxidative stress; thromboxane–prostanoid receptor; transforming growth factor-β.
Figures




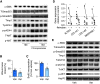
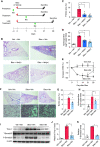
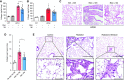
Comment in
-
From Biomarker to Mechanism? F2-isoprostanes in Pulmonary Fibrosis.Am J Respir Crit Care Med. 2022 Sep 1;206(5):530-532. doi: 10.1164/rccm.202205-0914ED. Am J Respir Crit Care Med. 2022. PMID: 35763804 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL092870/HL/NHLBI NIH HHS/United States
- R01 HL095797/HL/NHLBI NIH HHS/United States
- K08 HL130595/HL/NHLBI NIH HHS/United States
- R01HL119503/HL/NHLBI NIH HHS/United States
- P01HL108800/HL/NHLBI NIH HHS/United States
- K08HL130595/HL/NHLBI NIH HHS/United States
- R01HL145372/HL/NHLBI NIH HHS/United States
- T32HL094296/HL/NHLBI NIH HHS/United States
- T32 HL094296/HL/NHLBI NIH HHS/United States
- R01HL135011/HL/NHLBI NIH HHS/United States
- R01 HL119503/HL/NHLBI NIH HHS/United States
- R01 HL153246/HL/NHLBI NIH HHS/United States
- R01 HL145372/HL/NHLBI NIH HHS/United States
- K24HL143281/HL/NHLBI NIH HHS/United States
- R01HL151016/HL/NHLBI NIH HHS/United States
- K24 HL143281/HL/NHLBI NIH HHS/United States
- R01 HL135011/HL/NHLBI NIH HHS/United States
- P01HL092870/HL/NHLBI NIH HHS/United States
- P01 HL108800/HL/NHLBI NIH HHS/United States
- R01 HL151016/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

