Pevonedistat plus azacitidine vs azacitidine alone in higher-risk MDS/chronic myelomonocytic leukemia or low-blast-percentage AML
- PMID: 35728048
- PMCID: PMC9631625
- DOI: 10.1182/bloodadvances.2022007334
Pevonedistat plus azacitidine vs azacitidine alone in higher-risk MDS/chronic myelomonocytic leukemia or low-blast-percentage AML
Abstract
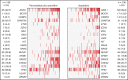
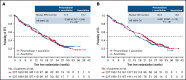
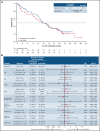
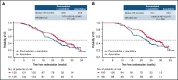
PANTHER is a global, randomized phase 3 trial of pevonedistat+azacitidine (n = 227) vs azacitidine monotherapy (n = 227) in patients with newly diagnosed higher-risk myelodysplastic syndromes (MDS; n = 324), higher-risk chronic myelomonocytic leukemia (n = 27), or acute myeloid leukemia (AML) with 20% to 30% blasts (n = 103). The primary end point was event-free survival (EFS). In the intent-to-treat population, the median EFS was 17.7 months with pevonedistat+azacitidine vs 15.7 months with azacitidine (hazard ratio [HR], 0.968; 95% confidence interval [CI], 0.757-1.238; P = .557) and in the higher-risk MDS cohort, median EFS was 19.2 vs 15.6 months (HR, 0.887; 95% CI, 0.659-1.193; P = .431). Median overall survival (OS) in the higher-risk MDS cohort was 21.6 vs 17.5 months (HR, 0.785; P = .092), and in patients with AML with 20% to 30% blasts was 14.5 vs 14.7 months (HR, 1.107; P = .664). In a post hoc analysis, median OS in the higher-risk MDS cohort for patients receiving >3 cycles was 23.8 vs 20.6 months (P = .021) and for >6 cycles was 27.1 vs 22.5 months (P = .008). No new safety signals were identified, and the azacitidine dose intensity was maintained. Common hematologic grade ≥3 treatment emergent adverse events were anemia (33% vs 34%), neutropenia (31% vs 33%), and thrombocytopenia (30% vs 30%). These results underscore the importance of large, randomized controlled trials in these heterogeneous myeloid diseases and the value of continuing therapy for >3 cycles. The trial was registered on clinicaltrials.gov as #NCT03268954.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures




References
-
- Granfeldt Østgård LS, Medeiros BC. Epidemiology and impact of preceding or underlying disease in secondary acute myeloid leukemia. HemaSphere. 2018;2(S2):153-155.
-
- Garcia-Manero G, Chien KS, Montalban-Bravo G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95(11):1399-1420. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

