The effect of various compounds on the COVID mechanisms, from chemical to molecular aspects
- PMID: 35728510
- PMCID: PMC9095071
- DOI: 10.1016/j.bpc.2022.106824
The effect of various compounds on the COVID mechanisms, from chemical to molecular aspects
Abstract
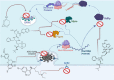
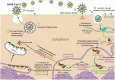
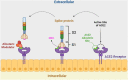
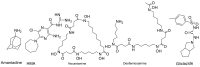
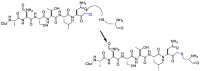
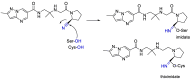
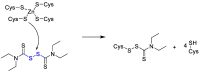
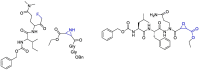
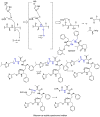
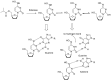
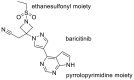
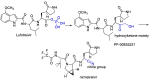
The novel coronavirus that caused COVID-19 pandemic is SARS-CoV-2. Although various vaccines are currently being used to prevent the disease's severe consequences, there is still a need for medications for those who become infected. The SARS-CoV-2 has a variety of proteins that have been studied extensively since the virus's advent. In this review article, we looked at chemical to molecular aspects of the various structures studied that have pharmaceutical activity and attempted to find a link between drug activity and compound structure. For example, designing of the compounds which bind to the allosteric site and modify hydrogen bonds or the salt bridges can disrupt SARS-CoV2 RBD-ACE2 complex. It seems that quaternary ammonium moiety and quinolin-1-ium structure could act as a negative allosteric modulator to reduce the tendency between spike-ACE2. Pharmaceutical structures with amino heads and hydrophobic tails can block envelope protein to prevent making mature SARS-CoV-2. Also, structures based on naphthalene pharmacophores or isosteres can form a strong bond with the PLpro and form a π-π and the Mpro's active site can be occupied by octapeptide compounds or linear compounds with a similar fitting ability to octapeptide compounds. And for protein RdRp, it is critical to consider pH and pKa so that pKa regulation of compounds to comply with patients is very effective, thus, the presence of tetrazole, phenylpyrazole groups, and analogs of pyrophosphate in the designed drugs increase the likelihood of the RdRp active site inhibition. Finally, it can be deduced that designing hybrid drug molecules along with considering the aforementioned characteristics would be a suitable approach for developing medicines in order to accurate targeting and complete inhibition this virus.
Keywords: 3Clpro; COVID-19; PLpro; RdRp; SARS-CoV-2; Spike-ACE2.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
None.
Figures
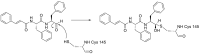
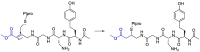
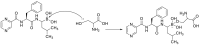
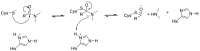
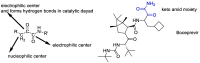
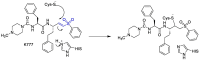
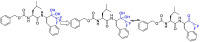
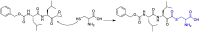
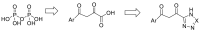
Similar articles
-
In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19.Comput Biol Med. 2020 Sep;124:103936. doi: 10.1016/j.compbiomed.2020.103936. Epub 2020 Jul 28. Comput Biol Med. 2020. PMID: 32738628 Free PMC article.
-
Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2.Sci Rep. 2020 Oct 19;10(1):17699. doi: 10.1038/s41598-020-74715-4. Sci Rep. 2020. PMID: 33077836 Free PMC article.
-
Dynamics of the ACE2-SARS-CoV-2/SARS-CoV spike protein interface reveal unique mechanisms.Sci Rep. 2020 Aug 26;10(1):14214. doi: 10.1038/s41598-020-71188-3. Sci Rep. 2020. PMID: 32848162 Free PMC article.
-
Inhibition of S-protein RBD and hACE2 Interaction for Control of SARSCoV- 2 Infection (COVID-19).Mini Rev Med Chem. 2021;21(6):689-703. doi: 10.2174/1389557520666201117111259. Mini Rev Med Chem. 2021. PMID: 33208074 Review.
-
COVID-19 Coronavirus spike protein analysis for synthetic vaccines, a peptidomimetic antagonist, and therapeutic drugs, and analysis of a proposed achilles' heel conserved region to minimize probability of escape mutations and drug resistance.Comput Biol Med. 2020 Jun;121:103749. doi: 10.1016/j.compbiomed.2020.103749. Epub 2020 Apr 11. Comput Biol Med. 2020. PMID: 32568687 Free PMC article. Review.
Cited by
-
Assessing the Potential Contribution of In Silico Studies in Discovering Drug Candidates That Interact with Various SARS-CoV-2 Receptors.Int J Mol Sci. 2023 Oct 24;24(21):15518. doi: 10.3390/ijms242115518. Int J Mol Sci. 2023. PMID: 37958503 Free PMC article. Review.
-
Potential RNA-dependent RNA polymerase (RdRp) inhibitors as prospective drug candidates for SARS-CoV-2.Eur J Med Chem. 2023 Apr 5;252:115292. doi: 10.1016/j.ejmech.2023.115292. Epub 2023 Mar 18. Eur J Med Chem. 2023. PMID: 36965227 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

