Metabolic cycles and signals for insulin secretion
- PMID: 35728586
- PMCID: PMC9262871
- DOI: 10.1016/j.cmet.2022.06.003
Metabolic cycles and signals for insulin secretion
Abstract
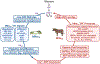
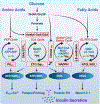
In this review, we focus on recent developments in our understanding of nutrient-induced insulin secretion that challenge a key aspect of the "canonical" model, in which an oxidative phosphorylation-driven rise in ATP production closes KATP channels. We discuss the importance of intrinsic β cell metabolic oscillations; the phasic alignment of relevant metabolic cycles, shuttles, and shunts; and how their temporal and compartmental relationships align with the triggering phase or the secretory phase of pulsatile insulin secretion. Metabolic signaling components are assigned regulatory, effectory, and/or homeostatic roles vis-à-vis their contribution to glucose sensing, signal transmission, and resetting the system. Taken together, these functions provide a framework for understanding how allostery, anaplerosis, and oxidative metabolism are integrated into the oscillatory behavior of the secretory pathway. By incorporating these temporal as well as newly discovered spatial aspects of β cell metabolism, we propose a much-refined MitoCat-MitoOx model of the signaling process for the field to evaluate.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
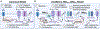


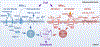

References
-
- Abulizi A, Cardone RL, Stark R, Lewandowski SL, Zhao X, Hillion J, Ma L, Sehgal R, Alves TC, Thomas C, Kung C, Wang B, Siebel S, Andrews ZB, Mason GF, Rinehart J, Merrins MJ, Kibbey RG, 2020. Multi-Tissue Acceleration of the Mitochondrial Phosphoenolpyruvate Cycle Improves Whole-Body Metabolic Health. Cell Metab 32, 751–766.e11. 10.1016/j.cmet.2020.10.006 - DOI - PMC - PubMed
-
- Adams MT, Dwulet JM, Briggs JK, Reissaus CA, Jin E, Szulczewski JM, Lyman MR, Sdao SM, Kravets V, Nimkulrat SD, Ponik SM, Merrins MJ, Mirmira RG, Linnemann AK, Benninger RK, Blum B, 2021. Reduced synchroneity of intra-islet Ca2+ oscillations in vivo in Robo-deficient β cells. Elife 10, e61308. 10.7554/eLife.61308 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

