Hypomagnesemia, Hypocalcemia, and Tubulointerstitial Nephropathy Caused by Claudin-16 Autoantibodies
- PMID: 35728884
- PMCID: PMC9257800
- DOI: 10.1681/ASN.2022010060
Hypomagnesemia, Hypocalcemia, and Tubulointerstitial Nephropathy Caused by Claudin-16 Autoantibodies
Abstract
Background: Chronic hypomagnesemia is commonly due to diarrhea, alcoholism, and drugs. More rarely, it is caused by genetic defects in the effectors of renal magnesium reabsorption.
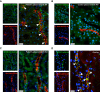
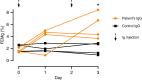
Methods: In an adult patient with acquired severe hypomagnesemia, hypocalcemia, tubulointerstitial nephropathy, and rapidly progressing kidney injury, similarities between the patient's presentation and features of genetic disorders of renal magnesium transport prompted us to investigate whether the patient had an acquired autoimmune cause of renal magnesium wasting. To determine if the patient's condition might be explained by autoantibodies directed against claudin-16 or claudin-19, transmembrane paracellular proteins involved in renal magnesium absorption, we conducted experiments with claudin knockout mice and transfected mouse kidney cells expressing human claudin-16 or claudin-19. We also examined effects on renal magnesium handling in rats given intravenous injections of IgG purified from sera from the patient or controls.
Results: Experiments with the knockout mice and in vitro transfected cells demonstrated that hypomagnesemia in the patient was causally linked to autoantibodies directed against claudin-16, which controls paracellular magnesium reabsorption in the thick ascending limb of Henle's loop. Intravenous injection of IgG purified from the patient's serum induced a marked urinary waste of magnesium in rats. Immunosuppressive treatment combining plasma exchange and rituximab was associated with improvement in the patient's GFR, but hypomagnesemia persisted. The patient was subsequently diagnosed with a renal carcinoma that expressed a high level of claudin-16 mRNA.
Conclusions: Pathogenic claudin-16 autoantibodies represent a novel autoimmune cause of specific renal tubular transport disturbances and tubulointerstitial nephropathy. Screening for autoantibodies targeting claudin-16, and potentially other magnesium transporters or channels in the kidney, may be warranted in patients with acquired unexplained hypomagnesemia.
Keywords: autoantibodies; claudin-16; hypocalcemia; hypomagnesemia.
Copyright © 2022 by the American Society of Nephrology.
Figures



Comment in
-
Autoimmune Renal Calcium and Magnesium Wasting.J Am Soc Nephrol. 2022 Jul;33(7):1231-1233. doi: 10.1681/ASN.2022050545. Epub 2022 Jun 21. J Am Soc Nephrol. 2022. PMID: 35728888 Free PMC article. No abstract available.
References
-
- de Baaij JH, Hoenderop JG, Bindels RJ: Magnesium in man: Implications for health and disease. Physiol Rev 95: 1–46, 2015 - PubMed
-
- Houillier P: Mechanisms and regulation of renal magnesium transport. Annu Rev Physiol 76: 411–430, 2014 - PubMed
-
- Olinger E, Houillier P, Devuyst O: Claudins: A tale of interactions in the thick ascending limb. Kidney Int 93: 535–537, 2018 - PubMed
-
- Agus ZS: Mechanisms and causes of hypomagnesemia. Curr Opin Nephrol Hypertens 25: 301–307, 2016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

