Cultured dissociated primary dorsal root ganglion neurons from adult horses enable study of axonal transport
- PMID: 35728923
- PMCID: PMC9558156
- DOI: 10.1111/joa.13719
Cultured dissociated primary dorsal root ganglion neurons from adult horses enable study of axonal transport
Abstract
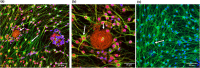
Neurological disorders are prevalent in horses, but their study is challenging due to anatomic constraints and the large body size; very few host-specific in vitro models have been established to study these types of diseases, particularly from adult donor tissue. Here we report the generation of primary neuronal dorsal root ganglia (DRG) cultures from adult horses: the mixed, dissociated cultures, containing neurons and glial cells, remained viable for at least 90 days. Similar to DRG neurons in vivo, cultured neurons varied in size, and they developed long neurites. The mitochondrial movement was detected in cultured cells and was significantly slower in glial cells compared to DRG-derived neurons. In addition, mitochondria were more elongated in glial cells than those in neurons. Our culture model will be a useful tool to study the contribution of axonal transport defects to specific neurodegenerative diseases in horses as well as comparative studies aimed at evaluating species-specific differences in axonal transport and survival.
Keywords: DRG; axonal transport; equine; mitochondria; neurodegenerative diseases.
© 2022 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures





References
-
- Aleman, M. , Williams, D.C. , Brosnan, R.J. , Nieto, J.E. , Pickles, K.J. , Berger, J. et al. (2013) Sensory nerve conduction and somatosensory evoked potentials of the trigeminal nerve in horses with idiopathic headshaking. Journal of Veterinary Internal Medicine, 27, 1571–1580. - PubMed
-
- Andrews, S. , Gilley, J. & Coleman, M.P. (2010) Difference Tracker: ImageJ plugins for fully automated analysis of multiple axonal transport parameters. Journal of Neuroscience Methods, 193, 281–287. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

