Human lungs show limited permissiveness for SARS-CoV-2 due to scarce ACE2 levels but virus-induced expansion of inflammatory macrophages
- PMID: 35728978
- PMCID: PMC9712848
- DOI: 10.1183/13993003.02725-2021
Human lungs show limited permissiveness for SARS-CoV-2 due to scarce ACE2 levels but virus-induced expansion of inflammatory macrophages
Erratum in
-
"Human lungs show limited permissiveness for SARS-CoV-2 due to scarce ACE2 levels but virus-induced expansion of inflammatory macrophages." K. Hönzke, B. Obermayer, C. Mache, et al. Eur Respir J 2022; 60: 2102725.Eur Respir J. 2023 Feb 9;61(2):2152725. doi: 10.1183/13993003.52725-2021. Print 2023 Feb. Eur Respir J. 2023. PMID: 36758997 Free PMC article. No abstract available.
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) utilises the angiotensin-converting enzyme 2 (ACE2) transmembrane peptidase as cellular entry receptor. However, whether SARS-CoV-2 in the alveolar compartment is strictly ACE2-dependent and to what extent virus-induced tissue damage and/or direct immune activation determines early pathogenesis is still elusive.
Methods: Spectral microscopy, single-cell/-nucleus RNA sequencing or ACE2 "gain-of-function" experiments were applied to infected human lung explants and adult stem cell derived human lung organoids to correlate ACE2 and related host factors with SARS-CoV-2 tropism, propagation, virulence and immune activation compared to SARS-CoV, influenza and Middle East respiratory syndrome coronavirus (MERS-CoV). Coronavirus disease 2019 (COVID-19) autopsy material was used to validate ex vivo results.
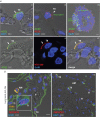
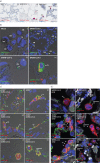
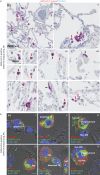
Results: We provide evidence that alveolar ACE2 expression must be considered scarce, thereby limiting SARS-CoV-2 propagation and virus-induced tissue damage in the human alveolus. Instead, ex vivo infected human lungs and COVID-19 autopsy samples showed that alveolar macrophages were frequently positive for SARS-CoV-2. Single-cell/-nucleus transcriptomics further revealed nonproductive virus uptake and a related inflammatory and anti-viral activation, especially in "inflammatory alveolar macrophages", comparable to those induced by SARS-CoV and MERS-CoV, but different from NL63 or influenza virus infection.
Conclusions: Collectively, our findings indicate that severe lung injury in COVID-19 probably results from a macrophage-triggered immune activation rather than direct viral damage of the alveolar compartment.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: J-C. Rückert and H. Radbruch report support from DFG RA 2491/1-1, BMBF (Defeat Pandemics). A.E. Hauser reports support from Charité – Universitätsmedizin Berlin and Deutsches Rheuma-Forschungszentrum Berlin, and grants from Deutsche Forschungsgemeinschaft (HA5354/10-1, TRR130,P17 and C01, HA5354/8-1). T. Wolff reports support from Federal Ministry of Education and Research (BMBF) grant 01K12006F. M. Kessler reports grants from BMBF Organo-Strat, Einstein 3R. M. Dohmen reports contracts with Max-Delbrück Center, Berlin; grants from Gender Equality Fund, Berlin Institute of Health. F. Klauschen reports consulting fees, lecture honoraria, travel support and participation on advisory boards with BMS, Novartis, Roche and Lilly, and is a co-founder of AI-BIH/Charité-Spinoff Aignostics GmbH. F. Heppner reports consulting fees, lecture honoraria, payment for expert testimony and leadership roles at Novartis, AstraZeneca and ThinkHealth Hygiene Solutions. V.M. Corman reports the following patents: 20210190797 (Methods and reagents for diagnosis of SARS-CoV-2 infection); 9841834 (Human recombinant monoclonal antibody against SARS-CoV-2 spike glycoprotein); 9909654 (A pharmaceutical combination comprising an anti-viral protonophore and a serine protease inhibitor). D. Niemeyer reports that Technische Universität Berlin, Freie Universität Berlin and Charité – Universitätsmedizin have filed a patent application for siRNAs inhibiting SARS-CoV-2 replication with D. Niemeyer as coauthor. M.A. Müller reports the following patents: 20210190797 (Methods and reagents for diagnosis of SARS-CoV-2 infection); 9841834 (Human recombinant monoclonal antibody against SARS-CoV-2 spike glycoprotein); 9909654 (A pharmaceutical combination comprising an anti-viral protonophore and a serine protease inhibitor); and has participated on an advisory board for ECDC/WHO. S. Ludwig reports consulting fees from Atriva Therapeutics GmbH, Biontec SE; and has patent PCT/EP2021/063485 pending. M. Witzenrath reports grants from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Deutsche Gesellschaft für Pneumologie, European Respiratory Society, Marie Curie Foundation, Else Kröner Fresenius Stiftung, Capnetz Stiftung, International Max Planck Research School, Quark Pharma, Takeda Pharma, Noxxon, Pantherna, Silence Therapeutics, Vaxxilon, Actelion, Bayer Health Care, Biotest and Boehringer Ingelheim; consulting fees from Noxxon, Pantherna, Silence Therapeutics, Vaxxilon, Aptarion, GlaxoSmithKline, Sinoxa and Biotest; lecture honoraria from AstraZeneca, Berlin Chemie, Chiesi, Novartis, Teva, Actelion, Boehringer Ingelheim, GlaxoSmithKline, Biotest, Bayer Health Care; and has the following patents issued: EPO 12181535.1 (IL-27 for modulation of immune response in acute lung injury), WO/2010/094491 (Means for inhibiting the expression of Ang-2), DE 102020116249.9 (Camostat/Niclosamide cotreatment in SARS-CoV-2 infected human lung cells). All other authors have nothing to disclose.
Figures




Comment in
-
Does autonomous macrophage-driven inflammation promote alveolar damage in COVID-19?Eur Respir J. 2022 Dec 1;60(6):2201521. doi: 10.1183/13993003.01521-2022. Print 2022 Dec. Eur Respir J. 2022. PMID: 36028257 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
