Basal Cell Carcinoma: A Narrative Review on Contemporary Diagnosis and Management
- PMID: 35729457
- PMCID: PMC9681969
- DOI: 10.1007/s40487-022-00201-8
Basal Cell Carcinoma: A Narrative Review on Contemporary Diagnosis and Management
Abstract
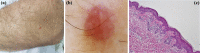
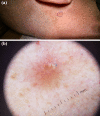
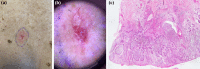
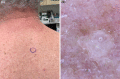
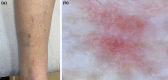
Basal cell carcinoma (BCC) is the most common, accounting for 80-90% of skin cancers. It arises from the basal layer of the epidermis and its appendages. A complex interplay of environmental, phenotypic and genetic variables leads to the development of BCC. Literature has documented several clinical subtypes of BCC, the most common of which are nodular, superficial and morpheaform. Expeditious diagnosis and analysis are essential for improving the outcome of BCC. Preventive measures, particularly when implemented in childhood and adolescence, may play a critical role. Due to its low metastatic potential, treatment for BCC mostly focuses on local management. The standard treatment of basal cell carcinoma involved complete removal of the lesion by excision or Mohs surgery. In special circumstances, basal cell carcinoma can be treated with cryosurgery, electrodesiccation and curettage, topical medications and photodynamic therapy. This review aimed to evaluate the contemporary diagnosis and management of basal cell carcinoma.
Keywords: Basal cell carcinoma; Diagnosis; Management; Surgical excision.
© 2022. The Author(s).
Figures

References
-
- The Skin Cancer Foundation. Skin Cancer Facts and statistics. [(accessed on 25 September 2021)]; Available online: http://www.skincancer.org/skin-cancer-information/skin-cancer-facts.
-
- Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol. 2017;177:359–372. - PubMed
-
- Walker H, Maitland C, Tabbakh T, Preston P, Wakefield M, Sinclair C. Forty years of Slip! Slop! Slap! A call to action on skin cancer prevention for Australia. Public Health Res Pract. 2022;32(1):1–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

