UFL1 alleviates ER stress and apoptosis stimulated by LPS via blocking the ferroptosis pathway in human granulosa-like cells
- PMID: 35729487
- PMCID: PMC9485362
- DOI: 10.1007/s12192-022-01284-y
UFL1 alleviates ER stress and apoptosis stimulated by LPS via blocking the ferroptosis pathway in human granulosa-like cells
Abstract
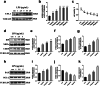
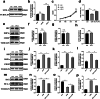
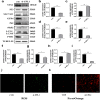
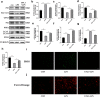
Ubiquitin-like modifier 1 ligating enzyme 1 (UFL1) is a unique E3 ligase of the UFMylation system. Recent studies have shown that this enzyme plays a crucial role in the processes of endoplasmic reticulum stress (ER stress) and apoptosis. Lipopolysaccharide (LPS) can cause injury to ovarian granule cells and hinder follicular development by triggering ER stress and apoptosis. Our study aimed to investigate the mechanism by which UFL1 alleviates ER stress and apoptosis caused by LPS in human granulosa-like cells (KGNs). In this study, we found that the protein levels of UFL1 were increased obviously under LPS stimulation in KGNs and that ER stress and apoptosis were further aggravated when UFL1 was knocked down; in contrast, these events were rescued when UFL1 was overexpressed. Next, we showed that the levels of ferroptosis-related proteins were relatively altered, accompanied by the accumulation of reactive oxygen species (ROS) and Fe2+, following the inhibition of UFL1 expression. In contrast, the overexpression of UFL1 reversed the ferroptosis process by regulating the P53/SLC7A11 (solute carrier family 7, member 11, SLC7A11) system and autophagy in response to LPS stimulation. Furthermore, apoptosis and ER stress in KGNs are rescued by the administration of the ferroptosis inhibitor ferrostatin-1 (Fer-1). Collectively, our research demonstrated a new mechanism for UFL1 that can alleviate ER stress and apoptosis stimulated by LPS; this occurred via the regulation of the ferroptosis pathway in KGNs and may provide a new strategy for research in the field of reproduction.
Keywords: Apoptosis; ER stress; Ferroptosis; UFL1.
© 2022. The Author(s), under exclusive licence to Cell Stress Society International.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Ubiquitin-like modifier 1 ligating enzyme 1 relieves cisplatin-induced premature ovarian failure by reducing endoplasmic reticulum stress in granulosa cells.Reprod Biol Endocrinol. 2022 May 24;20(1):84. doi: 10.1186/s12958-022-00956-9. Reprod Biol Endocrinol. 2022. PMID: 35610622 Free PMC article.
-
UFL1 Alleviates LPS-Induced Apoptosis by Regulating the NF-κB Signaling Pathway in Bovine Ovarian Granulosa Cells.Biomolecules. 2020 Feb 9;10(2):260. doi: 10.3390/biom10020260. Biomolecules. 2020. PMID: 32050508 Free PMC article.
-
UFL1 Alleviates Lipopolysaccharide-Induced Cell Damage and Inflammation via Regulation of the TLR4/NF-κB Pathway in Bovine Mammary Epithelial Cells.Oxid Med Cell Longev. 2019 Feb 10;2019:6505373. doi: 10.1155/2019/6505373. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30881595 Free PMC article.
-
Ufl1/RCAD, a Ufm1 E3 ligase, has an intricate connection with ER stress.Int J Biol Macromol. 2019 Aug 15;135:760-767. doi: 10.1016/j.ijbiomac.2019.05.170. Epub 2019 May 23. Int J Biol Macromol. 2019. PMID: 31129212 Review.
-
UFL1, a UFMylation E3 ligase, plays a crucial role in multiple cellular stress responses.Front Endocrinol (Lausanne). 2023 Feb 10;14:1123124. doi: 10.3389/fendo.2023.1123124. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36843575 Free PMC article. Review.
Cited by
-
Reduce electrical overload via threaded Chinese acupuncture in nerve electrical therapy.Bioact Mater. 2025 Jan 3;46:476-493. doi: 10.1016/j.bioactmat.2024.12.025. eCollection 2025 Apr. Bioact Mater. 2025. PMID: 39850020 Free PMC article.
-
UFMylation of NLRP3 Prevents Its Autophagic Degradation and Facilitates Inflammasome Activation.Adv Sci (Weinh). 2025 Apr;12(15):e2406786. doi: 10.1002/advs.202406786. Epub 2025 Feb 22. Adv Sci (Weinh). 2025. PMID: 39985286 Free PMC article.
-
Ferroptosis and endoplasmic reticulum stress in ischemic stroke.Neural Regen Res. 2024 Mar;19(3):611-618. doi: 10.4103/1673-5374.380870. Neural Regen Res. 2024. PMID: 37721292 Free PMC article. Review.
-
The emerging roles of UFMylation in the modulation of immune responses.Clin Transl Med. 2024 Sep;14(9):e70019. doi: 10.1002/ctm2.70019. Clin Transl Med. 2024. PMID: 39259506 Free PMC article. Review.
References
-
- Chen Y, Mi Y, Zhang X, Ma Q, Song Y, Zhang L, Wang D, Xing J, Hou B, Li H, Jin H, Du W, Zou Z. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J Exp Clin Cancer Res. 2019;38:402. doi: 10.1186/s13046-019-1413-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

