Xeno-free protocol for GMP-compliant manufacturing of human fetal pancreas-derived mesenchymal stem cells
- PMID: 35729640
- PMCID: PMC9210668
- DOI: 10.1186/s13287-022-02946-5
Xeno-free protocol for GMP-compliant manufacturing of human fetal pancreas-derived mesenchymal stem cells
Abstract
Background: Mesenchymal stem cells (MSCs) have been suggested as an appropriate source for diabetes cell-based therapies. The high proliferation and differentiation capacity of fetal MSCs and the role of fetal pancreatic-derived MSCs (FPMSCs) in islet generation make them good candidates for diabetes treatment. To manufacture clinical-grade MSCs, animal-free culture protocols are preferred. The current study aimed to establish a xeno-free/GMP-compliant protocol for FPMSCs manufacturing. The focus was on the effects of fetal bovine serum (FBS) replacement with pooled human serum (HS).
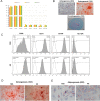
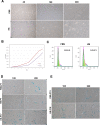
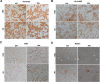
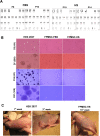
Material and methods: FPMSCs were isolated and expanded from the pancreas of legally aborted fetuses with few modifications in our previously established protocol. The cells were expanded in two different culture media, including DMEM supplemented with 10% FBS or 10% pooled HS. A side-by-side comparison was made to evaluate the effect of each serum on proliferation rate, cell cycle, senescence, multi-lineage differentiation capacity, immunophenotype, and tumorigenesis of FPMSCs.
Results: Flow cytometry analysis and three-lineage differentiation ability demonstrated that fibroblast-like cells obtained from primary culture had MSCs' characteristics. The FPMSCs displayed similar morphology and CD markers expression in both sera. HS had a higher proliferative effect on FPMSCs than FBS. In FBS, the cells reached senescence earlier. In addition to normal karyotypes and anchorage-dependent growth, in vivo tumor formation was not seen.
Conclusion: Our results demonstrated that HS was a better serum alternative than FBS for in vitro expansion of FPMSCs. Compared with FBS, HS increased FPMSCs' proliferation rate and decreased their senescence. In conclusion, HS can effectively replace FBS for clinical-grade FPMSCs manufacturing.
Keywords: Cell therapy; Diabetes; Fetal pancreas; GMP; Mesenchymal stem cell; Xeno-free.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

