Prediction and Preparation of Coamorphous Phases of a Bislactam
- PMID: 35730408
- PMCID: PMC9257746
- DOI: 10.1021/acs.molpharmaceut.2c00357
Prediction and Preparation of Coamorphous Phases of a Bislactam
Abstract
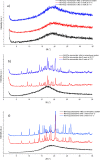
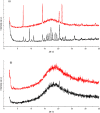
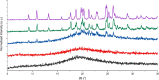
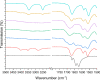
The effectiveness of a partial least squares-discriminant analysis coamorphous prediction model was tested using coamorphous screening data for a promising coamorphous former, the dimer of N-vinyl(caprolactam) (bisVCap) with a range of active pharmaceutical ingredients. The prediction model predicted 71% of the systems correctly. An experimental coamorphous screen was performed with this coformer with 13 different active pharmaceutical ingredients, and the results were compared to the predictions from the model. A total of 85% of the systems were correctly predicted. Stability assessments of three coamorphous systems showed that the prediction model score did not strongly correlate with the stability of the coamorphous material. The model performed well with small-molecule coformers, such as bisVCap, despite the difference in structure and properties compared to the amino-acid-based model training set.
Keywords: active pharmaceutical ingredients; bislactams; coamorphous; partial least squares-discriminant analysis; physical stability; predictive modeling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


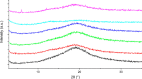
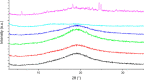
References
-
- Liu J.; Grohganz H.; Löbmann K.; Rades T.; Hempel N.-J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics 2021, 13, 389.10.3390/pharmaceutics13030389. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

