Biochemical, biophysical, and immunological characterization of respiratory secretions in severe SARS-CoV-2 infections
- PMID: 35730564
- PMCID: PMC9309048
- DOI: 10.1172/jci.insight.152629
Biochemical, biophysical, and immunological characterization of respiratory secretions in severe SARS-CoV-2 infections
Abstract
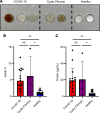
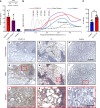
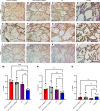
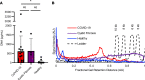
Thick, viscous respiratory secretions are a major pathogenic feature of COVID-19, but the composition and physical properties of these secretions are poorly understood. We characterized the composition and rheological properties (i.e., resistance to flow) of respiratory secretions collected from intubated COVID-19 patients. We found the percentages of solids and protein content were greatly elevated in COVID-19 compared with heathy control samples and closely resembled levels seen in cystic fibrosis, a genetic disease known for thick, tenacious respiratory secretions. DNA and hyaluronan (HA) were major components of respiratory secretions in COVID-19 and were likewise abundant in cadaveric lung tissues from these patients. COVID-19 secretions exhibited heterogeneous rheological behaviors, with thicker samples showing increased sensitivity to DNase and hyaluronidase treatment. In histologic sections from these same patients, we observed increased accumulation of HA and the hyaladherin versican but reduced tumor necrosis factor-stimulated gene-6 staining, consistent with the inflammatory nature of these secretions. Finally, we observed diminished type I interferon and enhanced inflammatory cytokines in these secretions. Overall, our studies indicated that increases in HA and DNA in COVID-19 respiratory secretion samples correlated with enhanced inflammatory burden and suggested that DNA and HA may be viable therapeutic targets in COVID-19 infection.
Keywords: COVID-19; Innate immunity; Pulmonology; Respiration.
Figures


Update of
-
Biochemical and Biophysical Characterization of Respiratory Secretions in Severe SARS-CoV-2 (COVID-19) Infections.medRxiv [Preprint]. 2021 Aug 19:2020.09.11.20191692. doi: 10.1101/2020.09.11.20191692. medRxiv. 2021. Update in: JCI Insight. 2022 Jun 22;7(12):e152629. doi: 10.1172/jci.insight.152629. PMID: 32935110 Free PMC article. Updated. Preprint.
-
Biochemical, Biophysical, and Immunological Characterization of Respiratory Secretions in Severe SARS-CoV-2 (COVID-19) Infections.medRxiv [Preprint]. 2022 Apr 4:2022.03.28.22272848. doi: 10.1101/2022.03.28.22272848. medRxiv. 2022. Update in: JCI Insight. 2022 Jun 22;7(12):e152629. doi: 10.1172/jci.insight.152629. PMID: 35411348 Free PMC article. Updated. Preprint.
Similar articles
-
Biochemical, Biophysical, and Immunological Characterization of Respiratory Secretions in Severe SARS-CoV-2 (COVID-19) Infections.medRxiv [Preprint]. 2022 Apr 4:2022.03.28.22272848. doi: 10.1101/2022.03.28.22272848. medRxiv. 2022. Update in: JCI Insight. 2022 Jun 22;7(12):e152629. doi: 10.1172/jci.insight.152629. PMID: 35411348 Free PMC article. Updated. Preprint.
-
Biochemical and Biophysical Characterization of Respiratory Secretions in Severe SARS-CoV-2 (COVID-19) Infections.medRxiv [Preprint]. 2021 Aug 19:2020.09.11.20191692. doi: 10.1101/2020.09.11.20191692. medRxiv. 2021. Update in: JCI Insight. 2022 Jun 22;7(12):e152629. doi: 10.1172/jci.insight.152629. PMID: 32935110 Free PMC article. Updated. Preprint.
-
SARS-CoV-2 infection induces hyaluronan production in vitro and hyaluronan levels in COVID-19 patients relate to morbidity and long-term lung impairment: a prospective cohort study.mBio. 2024 Oct 16;15(10):e0130324. doi: 10.1128/mbio.01303-24. Epub 2024 Sep 20. mBio. 2024. PMID: 39302125 Free PMC article.
-
Dysregulated Interferon Response and Immune Hyperactivation in Severe COVID-19: Targeting STATs as a Novel Therapeutic Strategy.Front Immunol. 2022 May 17;13:888897. doi: 10.3389/fimmu.2022.888897. eCollection 2022. Front Immunol. 2022. PMID: 35663932 Free PMC article. Review.
-
The role of hyaluronan synthesis and degradation in the critical respiratory illness COVID-19.Am J Physiol Cell Physiol. 2022 Jun 1;322(6):C1037-C1046. doi: 10.1152/ajpcell.00071.2022. Epub 2022 Apr 20. Am J Physiol Cell Physiol. 2022. PMID: 35442830 Free PMC article. Review.
Cited by
-
Ex-vivo mucolytic and anti-inflammatory activity of BromAc in tracheal aspirates from COVID-19.Biomed Pharmacother. 2022 Apr;148:112753. doi: 10.1016/j.biopha.2022.112753. Epub 2022 Feb 25. Biomed Pharmacother. 2022. PMID: 35272139 Free PMC article.
-
Physiotherapy management for COVID-19 in the acute hospital setting and beyond: an update to clinical practice recommendations.J Physiother. 2022 Jan;68(1):8-25. doi: 10.1016/j.jphys.2021.12.012. Epub 2021 Dec 23. J Physiother. 2022. PMID: 34953756 Free PMC article.
-
Clinical and prognostic implications of hyaluronic acid in patients with COVID-19 reinfection and first infection.Front Microbiol. 2024 May 31;15:1406581. doi: 10.3389/fmicb.2024.1406581. eCollection 2024. Front Microbiol. 2024. PMID: 38881657 Free PMC article.
-
Searching COVID-19 Clinical Research Using Graph Queries: Algorithm Development and Validation.J Med Internet Res. 2024 May 30;26:e52655. doi: 10.2196/52655. J Med Internet Res. 2024. PMID: 38814687 Free PMC article.
-
Hyaluronan in COVID-19: a matrix for understanding lung disease.mBio. 2024 Dec 11;15(12):e0260924. doi: 10.1128/mbio.02609-24. Epub 2024 Nov 18. mBio. 2024. PMID: 39555923 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

