Mycobiome Dysbiosis in Women with Intrauterine Adhesions
- PMID: 35730962
- PMCID: PMC9431258
- DOI: 10.1128/spectrum.01324-22
Mycobiome Dysbiosis in Women with Intrauterine Adhesions
Abstract
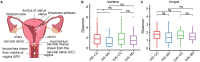
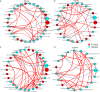
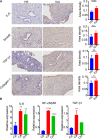
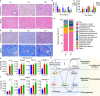
The vaginal microbiota dysbiosis is closely associated with the development of reproductive diseases. However, the contribution of mycobiome to intrauterine adhesion (IUA) disease remains unknown. Harnessing 16S and ITS2 rDNA sequencing analysis, we investigate both bacterial and fungal microbiota compositions across 174 samples taken from both cervical canal (CC) and middle vagina (MV) sites of IUA patients. Overall, there is no significant difference in microbial diversity between healthy subjects (HS) and IUA patients. However, we observe the IUA-specific bacterial alterations such as increased Dialister and decreased Bifidobacterium and enriched fungal genera like increased Filobasidium and Exophiala. Moreover, site-specific fungal-bacterial correlation networks are discovered in both CC and MV samples of IUA patients. Mechanistic investigation shows that Candida parapsilosis, other than Candida albicans and Candida maltosa, prevents the exacerbation of inflammatory activities and fibrosis, and modulates bacterial microbiota during IUA progression in a rat model of IUA. Our study thus highlights the importance of mycobiota in IUA progression, which may facilitate the development of therapeutic target for IUA prevention. IMPORTANCE Intrauterine adhesion (IUA) often leads to hypomenorrhea, amenorrhea, repeat miscarriages, and infertility. It has been prevalent over the last few decades in up to 13% of women who experience pregnancy termination during the first trimester, and 30% of women undergo dilation and curettage after a late, spontaneous abortion. However, the pathogenesis of IUA remains unclear. Despite reports of microbiota dysbiosis during IUA progression, there is little information on the effect of fungal microbiota on the development of IUA. This study not only enhances our understanding of the mycobiome in IUA patients but also provides potential intervention strategies for prevention of IUA by targeting mycobiome.
Keywords: dysbiosis; fungal-bacterial correlation; intrauterine adhesions; mycobiome; reproductive tract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wang YQ, Song XH, Wu SL, Huang YZ, Yan L, Li CZ. 2020. Comparison of autocross-linked hyaluronic acid gel and intrauterine device for preventing intrauterine adhesions in infertile patients: a randomized clinical trial. Gynecol Minim Invasive Ther 9:74–80. doi: 10.4103/GMIT.GMIT_103_19. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

