Impact of Fast SARS-CoV-2 Molecular Point-Of-Care Testing on Patients' Length of Stay in an Emergency Department
- PMID: 35730967
- PMCID: PMC9431206
- DOI: 10.1128/spectrum.00636-22
Impact of Fast SARS-CoV-2 Molecular Point-Of-Care Testing on Patients' Length of Stay in an Emergency Department
Abstract
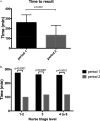
The ID NOW COVID-19 system (IDNOW) is a point-of-care test (POCT) providing results within 15 min. We evaluated the impact of IDNOW use on patient length of stay (LOS) in an emergency department (ED). In the ED of Saint-Louis Hospital, Paris, France, adult patients requiring a rapid diagnosis of SARS-CoV-2 were tested with Cepheid Xpert Xpress SARS-CoV-2 or FilmArray respiratory panel RP2 in the virology laboratory between 18 October and 3 November 2020 (period 1) and with IDNOW between 4 November and 30 November 2020 (period 2). A total of 676 patients participated in the study, 337 during period 1 and 339 during period 2. The median LOS in ED was significantly higher in period 1 than in period 2 (276 versus 208 min, P < 0.0001). More patients spent less than 4 h in the ED in period 2 (61.3%) than in period 1 (38.3%) (P < 0.0001). By univariate analysis, factors associated with ED LOS were hypertension, anosmia/ageusia, number of patients per day, and ID NOW implementation in period 2. By multivariate analysis, the period of testing remained significantly associated with ED LOS. Rapid molecular SARS-CoV-2 POCT was associated with a reduced LOS for patients admitted to an ED. IMPORTANCE During COVID-19 pandemic upsurges, emergency departments had to deal with a massive flow of incoming patients. The need for COVID-19 infection status determination before medical ward admission worsened ED overcrowding. The development of molecular point-of-care testing gave new opportunities for getting faster results of SARS-CoV-2 genome detection 24 h a day. In our study, we show, with a multivariate analysis, that the use of the POCT COVID-19 IDNOW reduced the ED length of stay by 1 h. The rate of patients who waited less than 4 h in the ED increased significantly. Our study highlights the benefit of COVID-19 molecular POCT for preventing ED overcrowding and facilitating bed allocation and SARS-CoV-2-infected patient isolation.
Keywords: COVID-19; SARS-CoV-2; length of stay; molecular detection; point of care.
Conflict of interest statement
The authors declare a conflict of interest. Jerome LeGoff received consulting fees from Abbott in 2021. Outside the submitted work, Jerome LeGoff reports consulting fees from bioMérieux and Roche Molecular (in 2018-2019); Constance Delaugerre reports to be member of a scientific board for MSD and Gilead ViiV, and a research grant from Gilead ViiV. All other authors declare no conflict of interest for the submitted work.
Figures

Similar articles
-
Clinical and operational impact of rapid point-of-care SARS-CoV-2 detection in an emergency department.Am J Emerg Med. 2021 Dec;50:713-718. doi: 10.1016/j.ajem.2021.09.062. Epub 2021 Sep 28. Am J Emerg Med. 2021. PMID: 34879491 Free PMC article.
-
Universal SARS-CoV-2 Testing of Emergency Department Admissions Increases Emergency Department Length of Stay.Ann Emerg Med. 2022 Feb;79(2):182-186. doi: 10.1016/j.annemergmed.2021.09.005. Epub 2021 Sep 8. Ann Emerg Med. 2022. PMID: 34756452 Free PMC article.
-
Added value of rapid respiratory syndromic testing at point of care versus central laboratory testing: a controlled clinical trial.J Antimicrob Chemother. 2021 Sep 23;76(Supplement_3):iii20-iii27. doi: 10.1093/jac/dkab241. J Antimicrob Chemother. 2021. PMID: 34555158 Free PMC article. Clinical Trial.
-
Point of care molecular and antigen detection tests for COVID-19: current status and future prospects.Expert Rev Mol Diagn. 2022 Aug;22(8):797-809. doi: 10.1080/14737159.2022.2122712. Epub 2022 Sep 14. Expert Rev Mol Diagn. 2022. PMID: 36093682 Review.
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
Cited by
-
Effects of Different SARS-CoV-2 Testing Strategies in the Emergency Department on Length of Stay and Clinical Outcomes: A Randomised Controlled Trial.Can J Infect Dis Med Microbiol. 2024 Feb 14;2024:9571236. doi: 10.1155/2024/9571236. eCollection 2024. Can J Infect Dis Med Microbiol. 2024. PMID: 38384429 Free PMC article.
-
Intensified screening for SARS-CoV-2 in 18 emergency departments in the Paris metropolitan area, France (DEPIST-COVID): A cluster-randomized, two-period, crossover trial.PLoS Med. 2023 Dec 7;20(12):e1004317. doi: 10.1371/journal.pmed.1004317. eCollection 2023 Dec. PLoS Med. 2023. PMID: 38060611 Free PMC article. Clinical Trial.
References
-
- Mustafa Hellou M, Górska A, Mazzaferri F, Cremonini E, Gentilotti E, De Nardo P, Poran I, Leeflang MM, Tacconelli E, Paul M. 2021. Nucleic acid amplification tests on respiratory samples for the diagnosis of coronavirus infections: a systematic review and meta-analysis. Clin Microbiol Infect 27:341–351. doi:10.1016/j.cmi.2020.11.002. - DOI - PMC - PubMed
-
- Hinson JS, Rothman RE, Carroll K, Mostafa HH, Ghobadi K, Smith A, Martinez D, Shaw-Saliba K, Klein E, Levin S. 2021. Targeted rapid testing for SARS-CoV-2 in the emergency department is associated with large reductions in uninfected patient exposure time. J Hosp Infect 107:35–39. doi:10.1016/j.jhin.2020.09.035. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

