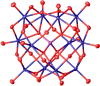
Spectroscopic, Crystallographic, and Electrochemical Study of Different Manganese(II)-Substituted Keggin-Type Phosphomolybdates
- PMID: 35731027
- PMCID: PMC9546069
- DOI: 10.1002/chem.202201084
Spectroscopic, Crystallographic, and Electrochemical Study of Different Manganese(II)-Substituted Keggin-Type Phosphomolybdates
Abstract
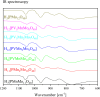
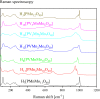
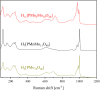
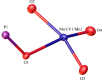
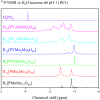
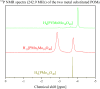
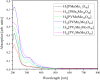
Adjusting the RedOx activity of polyoxometalate catalysts is a key challenge for the catalysis of selective oxidation reactions. For this purpose, the possibility of influencing the RedOx potential by the introduction of an additional RedOx-active element was investigated. Thereby, Keggin-type polyoxometalates (POMs) with up to three different elements in the metal framework were created. An advanced and reproducible synthetic procedure to incorporate MnII and additionally VV into Keggin-type heteropolyacids alongside comprehensive characterization of the new molecules is presented. The success of our syntheses was confirmed by vibrational spectroscopy (IR and Raman) and elemental analysis. Furthermore, the new compounds were analyzed by NMR spectroscopy to investigate the characteristics of the POMs in solution. The structures of successfully crystalized compounds were determined by single-crystal X-ray diffraction. Moreover, all synthesized compounds were characterized using UV/Vis spectroscopy and electrochemical analysis to get further insights into the electronic transfer processes and redox potentials.
Keywords: NMR spectroscopy; crystallography; electrochemistry; polyoxometalates; redox active elements.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Gumerova N. I., Rompel A., Nat. Chem. Rev. 2018, 2, 1–20.
-
- Lu F., Wang M., Li N., Tang B., Chem. Eur. J. 2021, 27, 6422–6434. - PubMed
-
- Yang L., Lei J., Fan J. M., Yuan R. M., Sen Zheng M., Chen J. J., Dong Q. F., Adv. Mater. 2021, 33, 1–25. - PubMed
-
- Song Y. F., Tsunashima R., Chem. Soc. Rev. 2012, 41, 7384–7402. - PubMed
-
- Neumann R., Inorg. Chem. 2010, 49, 3594–3601. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

