Simulation of COVID-19 symptoms in a genetically engineered mouse model: implications for the long haulers
- PMID: 35731343
- PMCID: PMC9214689
- DOI: 10.1007/s11010-022-04487-0
Simulation of COVID-19 symptoms in a genetically engineered mouse model: implications for the long haulers
Abstract
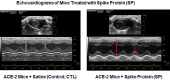
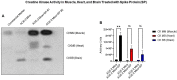
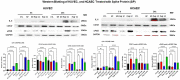
The ongoing pandemic (also known as coronavirus disease-19; COVID-19) by a constantly emerging viral agent commonly referred as the severe acute respiratory syndrome corona virus 2 or SARS-CoV-2 has revealed unique pathological findings from infected human beings, and the postmortem observations. The list of disease symptoms, and postmortem observations is too long to mention; however, SARS-CoV-2 has brought with it a whole new clinical syndrome in "long haulers" including dyspnea, chest pain, tachycardia, brain fog, exercise intolerance, and extreme fatigue. We opine that further improvement in delivering effective treatment, and preventive strategies would be benefited from validated animal disease models. In this context, we designed a study, and show that a genetically engineered mouse expressing the human angiotensin converting enzyme 2; ACE-2 (the receptor used by SARS-CoV-2 agent to enter host cells) represents an excellent investigative resource in simulating important clinical features of the COVID-19. The ACE-2 mouse model (which is susceptible to SARS-CoV-2) when administered with a recombinant SARS-CoV-2 spike protein (SP) intranasally exhibited a profound cytokine storm capable of altering the physiological parameters including significant changes in cardiac function along with multi-organ damage that was further confirmed via histological findings. More importantly, visceral organs from SP treated mice revealed thrombotic blood clots as seen during postmortem examination. Thus, the ACE-2 engineered mouse appears to be a suitable model for studying intimate viral pathogenesis thus paving the way for identification, and characterization of appropriate prophylactics as well as therapeutics for COVID-19 management.
Keywords: Clinical symptoms; Disease management; Humanized mouse; Multi-organ damage; SARS-CoV-2 spike protein.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no conflict of interest, financial or otherwise.
Figures



References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Singh M, Bhat PP, Mishra BP, Singh RK. Biological transmissibility of buffalopox virus. J Appl Anim Res. 1996;9:79–88. doi: 10.1080/09712119.1996.9706107. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

