Cortical-subcortical structural connections support transcranial magnetic stimulation engagement of the amygdala
- PMID: 35731882
- PMCID: PMC9217085
- DOI: 10.1126/sciadv.abn5803
Cortical-subcortical structural connections support transcranial magnetic stimulation engagement of the amygdala
Abstract
The amygdala processes valenced stimuli, influences emotion, and exhibits aberrant activity across anxiety disorders, depression, and PTSD. Interventions modulating amygdala activity hold promise as transdiagnostic psychiatric treatments. In 45 healthy participants, we investigated whether transcranial magnetic stimulation (TMS) elicits indirect changes in amygdala activity when applied to ventrolateral prefrontal cortex (vlPFC), a region important for emotion regulation. Harnessing in-scanner interleaved TMS/functional MRI (fMRI), we reveal that vlPFC neurostimulation evoked acute and focal modulations of amygdala fMRI BOLD signal. Larger TMS-evoked changes in the amygdala were associated with higher fiber density in a vlPFC-amygdala white matter pathway when stimulating vlPFC but not an anatomical control, suggesting this pathway facilitated stimulation-induced communication between cortex and subcortex. This work provides evidence of amygdala engagement by TMS, highlighting stimulation of vlPFC-amygdala circuits as a candidate treatment for transdiagnostic psychopathology. More broadly, it indicates that targeting cortical-subcortical structural connections may enhance the impact of TMS on subcortical neural activity and, by extension, subcortex-subserved behaviors.
Figures
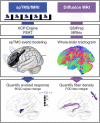
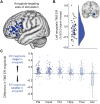
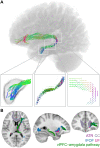

References
-
- Gründemann J., Bitterman Y., Lu T., Krabbe S., Grewe B. F., Schnitzer M. J., Lüthi A., Amygdala ensembles encode behavioral states. Science 364, eaav8736 (2019). - PubMed
-
- Agustín-Pavón C., Braesicke K., Shiba Y., Santangelo A. M., Mikheenko Y., Cockroft G., Asma F., Clarke H., Man M.-S., Roberts A. C., Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biol. Psychiatry 72, 266–272 (2012). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

