Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants
- PMID: 35731908
- PMCID: PMC9342588
- DOI: 10.1056/NEJMoa2204399
Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants
Abstract
Background: Infants younger than 6 months of age are at high risk for complications of coronavirus disease 2019 (Covid-19) and are not eligible for vaccination. Transplacental transfer of antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after maternal Covid-19 vaccination may confer protection against Covid-19 in infants.
Methods: We used a case-control test-negative design to assess the effectiveness of maternal vaccination during pregnancy against hospitalization for Covid-19 among infants younger than 6 months of age. Between July 1, 2021, and March 8, 2022, we enrolled infants hospitalized for Covid-19 (case infants) and infants hospitalized without Covid-19 (control infants) at 30 hospitals in 22 states. We estimated vaccine effectiveness by comparing the odds of full maternal vaccination (two doses of mRNA vaccine) among case infants and control infants during circulation of the B.1.617.2 (delta) variant (July 1, 2021, to December 18, 2021) and the B.1.1.259 (omicron) variant (December 19, 2021, to March 8, 2022).
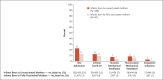
Results: A total of 537 case infants (181 of whom had been admitted to a hospital during the delta period and 356 during the omicron period; median age, 2 months) and 512 control infants were enrolled and included in the analyses; 16% of the case infants and 29% of the control infants had been born to mothers who had been fully vaccinated against Covid-19 during pregnancy. Among the case infants, 113 (21%) received intensive care (64 [12%] received mechanical ventilation or vasoactive infusions). Two case infants died from Covid-19; neither infant's mother had been vaccinated during pregnancy. The effectiveness of maternal vaccination against hospitalization for Covid-19 among infants was 52% (95% confidence interval [CI], 33 to 65) overall, 80% (95% CI, 60 to 90) during the delta period, and 38% (95% CI, 8 to 58) during the omicron period. Effectiveness was 69% (95% CI, 50 to 80) when maternal vaccination occurred after 20 weeks of pregnancy and 38% (95% CI, 3 to 60) during the first 20 weeks of pregnancy.
Conclusions: Maternal vaccination with two doses of mRNA vaccine was associated with a reduced risk of hospitalization for Covid-19, including for critical illness, among infants younger than 6 months of age. (Funded by the Centers for Disease Control and Prevention.).
Copyright © 2022 Massachusetts Medical Society.
Figures


Comment in
-
Covid-19 Vaccination during Pregnancy - Two for the Price of One.N Engl J Med. 2022 Jul 14;387(2):178-179. doi: 10.1056/NEJMe2206730. Epub 2022 Jun 22. N Engl J Med. 2022. PMID: 35731898 Free PMC article. No abstract available.
References
-
- Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021;27:1693-1695 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
