Pharmacokinetic-Pharmacodynamic Determinants of Clinical Outcomes for Rifampin-Resistant Tuberculosis: A Multisite Prospective Cohort Study
- PMID: 35731948
- PMCID: PMC9907514
- DOI: 10.1093/cid/ciac511
Pharmacokinetic-Pharmacodynamic Determinants of Clinical Outcomes for Rifampin-Resistant Tuberculosis: A Multisite Prospective Cohort Study
Abstract
Background: Rifampin-resistant and/or multidrug-resistant tuberculosis (RR/MDR-TB) treatment requires multiple drugs, and outcomes remain suboptimal. Some drugs are associated with improved outcome. It is unknown whether particular pharmacokinetic-pharmacodynamic relationships predict outcome.
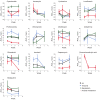
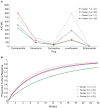
Methods: Adults with pulmonary RR/MDR-TB in Tanzania, Bangladesh, and the Russian Federation receiving local regimens were enrolled from June 2016 to July 2018. Serum was collected after 2, 4, and 8 weeks for each drug's area under the concentration-time curve over 24 hours (AUC0-24). Quantitative susceptibility of the M. tuberculosis isolate was measured by minimum inhibitory concentrations (MICs). Individual drug AUC0-24/MIC targets were assessed by adjusted odds ratios (ORs) for favorable treatment outcome, and hazard ratios (HRs) for time to sputum culture conversion. K-means clustering algorithm separated the cohort of the most common multidrug regimen into 4 clusters by AUC0-24/MIC exposures.
Results: Among 290 patients, 62 (21%) experienced treatment failure, including 30 deaths. Moxifloxacin AUC0-24/MIC target of 58 was associated with favorable treatment outcome (OR, 3.75; 95% confidence interval, 1.21-11.56; P = .022); levofloxacin AUC0-24/MIC of 118.3, clofazimine AUC0-24/MIC of 50.5, and pyrazinamide AUC0-24 of 379 mg × h/L were associated with faster culture conversion (HR >1.0, P < .05). Other individual drug exposures were not predictive. Clustering by AUC0-24/MIC revealed that those with the lowest multidrug exposures had the slowest culture conversion.
Conclusions: Amidst multidrug regimens for RR/MDR-TB, serum pharmacokinetics and M. tuberculosis MICs were variable, yet defined parameters to certain drugs-fluoroquinolones, pyrazinamide, clofazimine-were predictive and should be optimized to improve clinical outcome.
Clinical trials registration: NCT03559582.
Keywords: minimum inhibitory concentrations; multidrug-resistant tuberculosis; pharmacodynamics; pharmacokinetics.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. S. K. H., C. A. P., and E. R. H. report grants or contracts from the NIH outside of the submitted work. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- World Health Organization . Global tuberculosis report. Geneva, Switzerland: World Health Organization, 2021.
-
- Schnippel K, Firnhaber C, Ndjeka N, et al. . Persistently high early mortality despite rapid diagnostics for drug-resistant tuberculosis cases in South Africa. Int J Tuberc Lung Dis 2017; 21:1106–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

