Shielding Protection by Mesoporous Catalysts for Improving Plasma-Catalytic Ambient Ammonia Synthesis
- PMID: 35731953
- PMCID: PMC9284550
- DOI: 10.1021/jacs.2c01950
Shielding Protection by Mesoporous Catalysts for Improving Plasma-Catalytic Ambient Ammonia Synthesis
Abstract
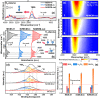
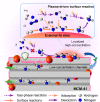
Plasma catalysis is a promising technology for decentralized small-scale ammonia (NH3) synthesis under mild conditions using renewable energy, and it shows great potential as an alternative to the conventional Haber-Bosch process. To date, this emerging process still suffers from a low NH3 yield due to a lack of knowledge in the design of highly efficient catalysts and the in situ plasma-induced reverse reaction (i.e., NH3 decomposition). Here, we demonstrate that a bespoke design of supported Ni catalysts using mesoporous MCM-41 could enable efficient plasma-catalytic NH3 production at 35 °C and 1 bar with >5% NH3 yield at 60 kJ/L. Specifically, the Ni active sites were deliberately deposited on the external surface of MCM-41 to enhance plasma-catalyst interactions and thus NH3 production. The desorbed NH3 could then diffuse into the ordered mesopores of MCM-41 to be shielded from decomposition due to the absence of plasma discharge in the mesopores of MCM-41, that is, "shielding protection", thus driving the reaction forward effectively. This promising strategy sheds light on the importance of a rational design of catalysts specifically for improving plasma-catalytic processes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Vojvodic A.; Medford A. J.; Studt F.; Abild-Pedersen F.; Khan T. S.; Bligaard T.; Nørskov J. K. Exploring the Limits: A Low-Pressure, Low-Temperature Haber-Bosch Process. Chem. Phys. Lett. 2014, 598, 108–112. 10.1016/j.cplett.2014.03.003. - DOI
-
- Guo J.; Chen P. Ammonia History in the Making. Nat. Catal. 2021, 4, 734–735. 10.1038/s41929-021-00676-0. - DOI
-
- Kyriakou V.; Garagounis I.; Vourros A.; Vasileiou E.; Stoukides M. An Electrochemical Haber-Bosch Process. Joule 2020, 4, 142–158. 10.1016/j.joule.2019.10.006. - DOI
LinkOut - more resources
Full Text Sources

