Preferential Regulation of Transient Protein-Protein Interaction by the Macromolecular Crowders
- PMID: 35731981
- PMCID: PMC9272825
- DOI: 10.1021/acs.jpcb.2c02713
Preferential Regulation of Transient Protein-Protein Interaction by the Macromolecular Crowders
Abstract
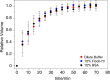
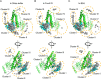
The environmental condition is a critical regulation factor for protein behavior in solution. Several studies have shown that macromolecular crowders can modulate protein structures, interactions, and functions. Recent publications described the regulation of specific interaction by macromolecular crowders. However, the other category of protein-protein interaction, namely, the transient interaction, is rarely investigated, especially from the perspective of protein structure to study transient interactions between proteins. Here, we used nuclear magnetic resonance and small-angle X-ray/neutron scattering methods to structurally investigate the ensemble of the protein complex in dilute buffer and crowded environments. Histidine phosphocarrier protein (HPr) and the N-terminal domain of enzyme I (EIN) are the important components of the bacterial phosphotransfer system. Our results show that the addition of Ficoll-70 promotes HPr molecules to form the encounter complex with EIN maintained by long-range electrostatic interaction. However, when macromolecular crowder BSA is used, the soft interaction between BSA and HPr perturbs the active site of HPr, driving HPr to form an encounter complex with EIN at the weakly charged interface. Our results indicate that different macromolecular crowders could influence transient EIN-HPr interaction through different mechanisms and provide new insights into protein-protein interaction regulation in native environments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Zhou Y. L.; Liao J. M.; Chen J.; Liang Y. Macromolecular crowding enhances the binding of superoxide dismutase to xanthine oxidase: implications for protein-protein interactions in intracellular environments. Int. J. Biochem. Cell Biol. 2006, 38, 1986–1994. 10.1016/j.biocel.2006.05.012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

